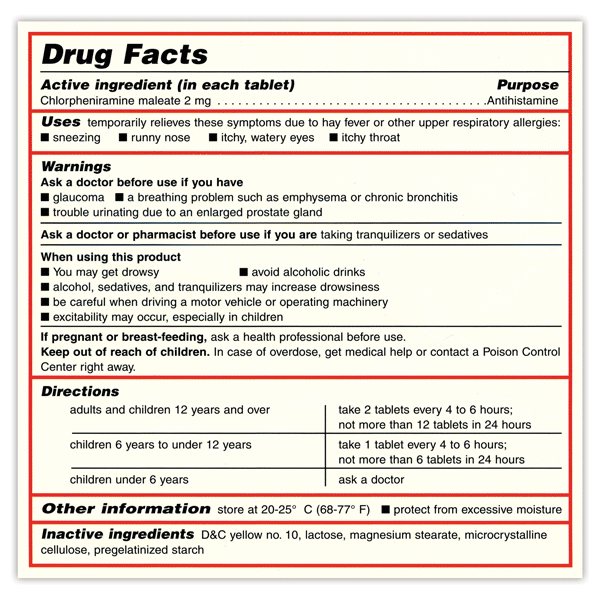
Fda Drug Label Guidance . Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Search for labels on dailymed. Fda's prescription drug labeling resources. This guidance is intended to assist applicants of human prescription drug and biological products in determining the.
from www.fda.gov
Fda's prescription drug labeling resources. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug.
OTC Drug Facts Label FDA
Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. Fda's prescription drug labeling resources. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. The labels are also available on the national library of medicine's dailymed web site.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Drug Label Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda's. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda's. Fda Drug Label Guidance.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Human prescription drug labeling (1) contains. Fda Drug Label Guidance.
From www.finnegan.com
Final FDA Guidance on Safety Considerations for Medication Container Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda's prescription drug labeling resources. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Search for labels on dailymed. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a. Fda Drug Label Guidance.
From thepointjournal.org
Point 74 “FDA Approved” Let’s Get Real Pat McCarthy The Point Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Search for labels on dailymed. The. Fda Drug Label Guidance.
From www.vecteezy.com
FDA Approved Food and Drug Administration stamp, icon, symbol, label Fda Drug Label Guidance The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Search for labels on dailymed. Fda's prescription drug labeling resources.. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants. Fda Drug Label Guidance.
From www.fda.gov
Sample Drug Facts Label FDA Fda Drug Label Guidance This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Fda's prescription drug labeling resources. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. The labels are also available on the national library of medicine's dailymed web site. This guidance is intended. Fda Drug Label Guidance.
From www.vrogue.co
Sample Drug Facts Label Fda vrogue.co Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda's prescription drug labeling resources.. Fda Drug Label Guidance.
From www.pm360online.com
What Does the FDA’s New Offlabel Guidance Mean for Pharma? PM360 Fda Drug Label Guidance The labels are also available on the national library of medicine's dailymed web site. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. This guidance is intended to assist applicants in. Fda Drug Label Guidance.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. The labels are also available on the national library of medicine's dailymed web site. Fda's prescription drug labeling resources. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended. Fda Drug Label Guidance.
From www.lifealert.org
OvertheCounter Medicine Label Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. The labels are also available on the national library of medicine's dailymed web site. Search for labels on dailymed. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants. Fda Drug Label Guidance.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Drug Label Guidance The labels are also available on the national library of medicine's dailymed web site. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda's prescription drug labeling resources. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. This guidance is intended to assist. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance The labels are also available on the national library of medicine's dailymed web site. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda is issuing this guidance to provide recommendations for applicants. Fda Drug Label Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Drug Label Guidance Search for labels on dailymed. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Fda's prescription drug labeling resources. The labels are also available on the national library of medicine's dailymed web site. This guidance. Fda Drug Label Guidance.
From de.slideshare.net
Fda proposes new guide for drug labeling Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search for labels on dailymed. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug,. Fda Drug Label Guidance.
From www.fda.gov
OTC Drug Facts Label FDA Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug,. Fda Drug Label Guidance.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Search for labels on. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Fda is issuing this guidance to provide recommendations for applicants developing labeling for. Fda Drug Label Guidance.
From www.statnews.com
FDA’s plan to define 'healthy' for food packaging Do we really need it? Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Search for labels on dailymed. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda's. Fda Drug Label Guidance.
From www.slideshare.net
Fda proposes new guide for drug labeling Fda Drug Label Guidance Search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda's prescription drug labeling resources. This guidance is intended to assist applicants of human prescription drug and biological products in. Fda Drug Label Guidance.
From nutrition.ftempo.com
Fda Nutrition Label Guidelines 2017 Nutrition Ftempo Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Search for labels on dailymed. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug.. Fda Drug Label Guidance.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Label Guidance For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda's prescription. Fda Drug Label Guidance.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Drug Label Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Search for labels on dailymed. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. This guidance. Fda Drug Label Guidance.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Drug Label Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda's prescription drug labeling resources. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription. Fda Drug Label Guidance.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Drug Label Guidance This guidance is intended to assist applicants in preparing the clinical pharmacology section of prescription drug. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. The labels are also available. Fda Drug Label Guidance.
From www.medscape.com
Introduction to the New Prescription Drug Labeling by the FDA Fda Drug Label Guidance For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance. Fda Drug Label Guidance.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Fda Drug Label Guidance This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. For purposes of prescription drug labeling,. Fda Drug Label Guidance.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Drug Label Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. The labels are also available on the national library of medicine's dailymed web site. Fda's prescription drug labeling resources. This. Fda Drug Label Guidance.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Drug Label Guidance For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. This guidance is intended. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance Fda's prescription drug labeling resources. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. Search for labels on dailymed. Fda is issuing this guidance to provide recommendations for applicants developing labeling. Fda Drug Label Guidance.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Fda Drug Label Guidance For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance is intended to assist applicants of human prescription drug and biological products in determining the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda's prescription drug. Fda Drug Label Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Label Guidance Fda is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising. The labels are also available on the national library of medicine's dailymed web site. Search for labels on dailymed. For purposes of prescription drug labeling, an adverse reaction is an undesirable effect, reasonably associated with use of a drug, that. This guidance. Fda Drug Label Guidance.