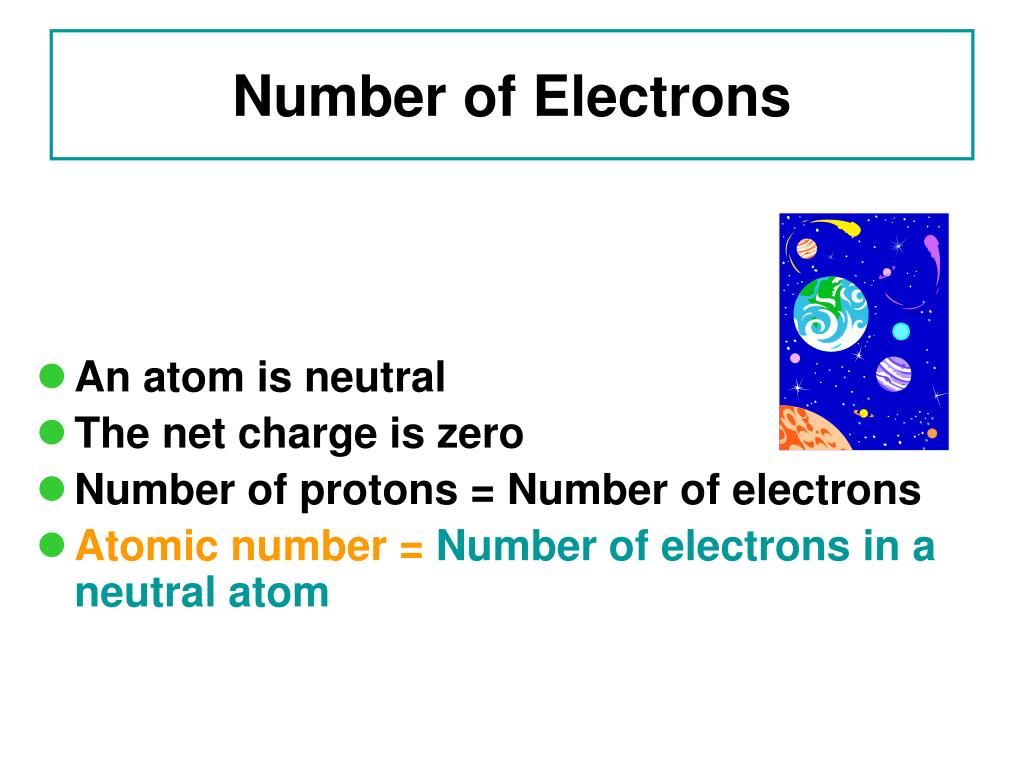
How Do You Work Out Electrons In An Atom . You can determine the number of electrons in an ion if you know. Therefore, you can determine the number of protons if the. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Knowing how to find the number of valence electrons in. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Number of protons = number of electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. For a neutral atom, the number of protons and the number of electrons are equal. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and.
from www.slideserve.com
You can determine the number of electrons in an ion if you know. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Knowing how to find the number of valence electrons in. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For a neutral atom, the number of protons and the number of electrons are equal. Therefore, you can determine the number of protons if the.
PPT Summary of the Atom PowerPoint Presentation, free download ID
How Do You Work Out Electrons In An Atom Describe the locations, charges, and masses of the three main subatomic particles. You can determine the number of electrons in an ion if you know. Determine the number of protons and electrons. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. For a neutral atom, the number of protons and the number of electrons are equal. Therefore, you can determine the number of protons if the. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Describe the locations, charges, and masses of the three main subatomic particles. Knowing how to find the number of valence electrons in. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Number of protons = number of electrons.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. Number of protons = number of electrons. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. You can use these numbers to calculate the. How Do You Work Out Electrons In An Atom.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons How Do You Work Out Electrons In An Atom Describe the locations, charges, and masses of the three main subatomic particles. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Knowing how to find the number of valence electrons in. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in. How Do You Work Out Electrons In An Atom.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Determine the number of protons and electrons. Knowing how to find the number of valence electrons in. Often, the number of protons and electrons is not the same, so. How Do You Work Out Electrons In An Atom.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram How Do You Work Out Electrons In An Atom Number of protons = number of electrons. You can determine the number of electrons in an ion if you know. Describe the locations, charges, and masses of the three main subatomic particles. Knowing how to find the number of valence electrons in. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons,. How Do You Work Out Electrons In An Atom.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Do You Work Out Electrons In An Atom This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Determine the number of protons and electrons. You can determine the number of electrons in an ion if you know. For a neutral atom, the number of protons and the number of electrons are equal. Describe the locations, charges, and masses of the three main subatomic. How Do You Work Out Electrons In An Atom.
From sciencenotes.org
List of Electron Configurations of Elements How Do You Work Out Electrons In An Atom You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. You can determine the number of electrons in an ion if you know. Determine the number of protons and electrons. For a neutral atom, the number of protons and the number of electrons are equal. Number of protons = number of electrons. In. How Do You Work Out Electrons In An Atom.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram How Do You Work Out Electrons In An Atom In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Number of protons = number of electrons. Knowing how to find the number of valence electrons in. You can determine the number of electrons in an ion if you know. For a neutral atom, the. How Do You Work Out Electrons In An Atom.
From www.broadlearnings.com
Atomic Structure Broad Learnings How Do You Work Out Electrons In An Atom For a neutral atom, the number of protons and the number of electrons are equal. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Therefore, you can determine the number of protons if the.. How Do You Work Out Electrons In An Atom.
From wiringguidefrosts.z19.web.core.windows.net
Electron Orbital Diagram Chart How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. Number of protons = number of electrons. Determine the number of protons and electrons. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. In this. How Do You Work Out Electrons In An Atom.
From www.youtube.com
The Number of Protons, Neutrons and Electrons YouTube How Do You Work Out Electrons In An Atom You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Therefore, you can determine the number of protons if the. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Often, the number of protons and electrons is not the same, so the atom carries. How Do You Work Out Electrons In An Atom.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by How Do You Work Out Electrons In An Atom Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. For a neutral atom, the number of protons and the number of electrons are equal. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Determine the number of protons and electrons.. How Do You Work Out Electrons In An Atom.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons How Do You Work Out Electrons In An Atom This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Therefore, you can determine the number of protons if the. Number of protons = number of electrons. Knowing how to find the number of valence electrons in. For a neutral atom, the number of protons and the number of electrons are equal. Describe the locations, charges,. How Do You Work Out Electrons In An Atom.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. Knowing how to find the number of valence electrons in. Determine the number of protons and electrons. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Describe the locations, charges, and masses of the three main subatomic particles. This. How Do You Work Out Electrons In An Atom.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out Electrons In An Atom Describe the locations, charges, and masses of the three main subatomic particles. For a neutral atom, the number of protons and the number of electrons are equal. Therefore, you can determine the number of protons if the. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Knowing how to find the number. How Do You Work Out Electrons In An Atom.
From www.slideserve.com
PPT Summary of the Atom PowerPoint Presentation, free download ID How Do You Work Out Electrons In An Atom This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Knowing how to find the number of valence electrons in. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element For a neutral atom, the number of protons and the number. How Do You Work Out Electrons In An Atom.
From www.animalia-life.club
Electrons In An Atom How Do You Work Out Electrons In An Atom Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. You can determine the number of electrons in an ion if you know. Knowing how to find the number of valence electrons in. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three. How Do You Work Out Electrons In An Atom.
From www.animalia-life.club
Electrons In An Atom How Do You Work Out Electrons In An Atom In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Therefore, you can determine the number of protons if the. You can determine the number of electrons in an ion if you know. Number of protons = number of electrons. Determine the number of protons and electrons. In this chemtalk tutorial, you. How Do You Work Out Electrons In An Atom.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. In this chemtalk tutorial, you will learn how to easily calculate and find the number. How Do You Work Out Electrons In An Atom.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram How Do You Work Out Electrons In An Atom Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Describe the locations, charges,. How Do You Work Out Electrons In An Atom.
From www.animalia-life.club
Electrons In An Atom How Do You Work Out Electrons In An Atom Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Therefore, you can determine the number of protons if the. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. You can determine the number of electrons in an ion if. How Do You Work Out Electrons In An Atom.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science How Do You Work Out Electrons In An Atom Determine the number of protons and electrons. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Number of protons = number of electrons. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Therefore, you can determine the number of protons if the. In. How Do You Work Out Electrons In An Atom.
From slidetodoc.com
Introduction to Basic Chemistry Protons Neutrons Electrons and How Do You Work Out Electrons In An Atom In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Knowing how to find the number of valence electrons in. For a neutral atom, the number of protons and the number of electrons are equal. Describe the locations, charges, and masses of the three main subatomic particles. Therefore, you can determine the. How Do You Work Out Electrons In An Atom.
From www.youtube.com
How to work out the electron configuration of an element YouTube How Do You Work Out Electrons In An Atom Therefore, you can determine the number of protons if the. For a neutral atom, the number of protons and the number of electrons are equal. Knowing how to find the number of valence electrons in. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Describe the locations, charges,. How Do You Work Out Electrons In An Atom.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. Describe the locations, charges, and masses of the three main subatomic particles. Therefore, you can determine the number of protons if the. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element. How Do You Work Out Electrons In An Atom.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science How Do You Work Out Electrons In An Atom Therefore, you can determine the number of protons if the. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. For a neutral atom, the number of. How Do You Work Out Electrons In An Atom.
From www.youtube.com
Electron Configuration of Atoms + Shortcut Tutorial Video YouTube How Do You Work Out Electrons In An Atom Determine the number of protons and electrons. You can determine the number of electrons in an ion if you know. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. This chemistry. How Do You Work Out Electrons In An Atom.
From mmerevise.co.uk
Atoms Questions and Revision MME How Do You Work Out Electrons In An Atom In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Knowing how to find the number of valence electrons in. This chemistry video tutorial explains how to. How Do You Work Out Electrons In An Atom.
From www.animalia-life.club
Electron Shell Diagram How Do You Work Out Electrons In An Atom Describe the locations, charges, and masses of the three main subatomic particles. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Often, the number of protons and. How Do You Work Out Electrons In An Atom.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry How Do You Work Out Electrons In An Atom Therefore, you can determine the number of protons if the. For a neutral atom, the number of protons and the number of electrons are equal. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Describe the locations, charges, and masses of the three main subatomic particles. In this chemtalk tutorial, you. How Do You Work Out Electrons In An Atom.
From www.youtube.com
Calculate the number of protons, neutrons and electrons in 80Br35 How Do You Work Out Electrons In An Atom In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Describe the locations, charges, and masses of the three main subatomic particles. You can determine the number of electrons in an ion if you know. Therefore, you can determine the number of protons if the. Knowing how to find the number of. How Do You Work Out Electrons In An Atom.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Do You Work Out Electrons In An Atom Determine the number of protons and electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. For a neutral atom, the number of protons and the number of electrons are equal. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons,. How Do You Work Out Electrons In An Atom.
From www.youtube.com
A Simle Guide to Finding the Numbers of Neutrons, Protons and Electrons How Do You Work Out Electrons In An Atom Determine the number of protons and electrons. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. For a neutral atom, the number of protons and the number of electrons are equal. You can determine. How Do You Work Out Electrons In An Atom.
From sites.google.com
Structure of the Atom Unit G Review How Do You Work Out Electrons In An Atom You can determine the number of electrons in an ion if you know. In chemistry, valence electrons are the electrons that are located in the outermost electron shell of an element. Describe the locations, charges, and masses of the three main subatomic particles. Knowing how to find the number of valence electrons in. This chemistry video tutorial explains how to. How Do You Work Out Electrons In An Atom.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Do You Work Out Electrons In An Atom Knowing how to find the number of valence electrons in. You can determine the number of electrons in an ion if you know. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Therefore, you can determine the number of protons if the. You can. How Do You Work Out Electrons In An Atom.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out Electrons In An Atom Knowing how to find the number of valence electrons in. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Describe the locations, charges, and masses of the three main subatomic particles. For a neutral atom, the number of protons and the number of electrons are equal. Number of protons = number of electrons. Therefore, you. How Do You Work Out Electrons In An Atom.