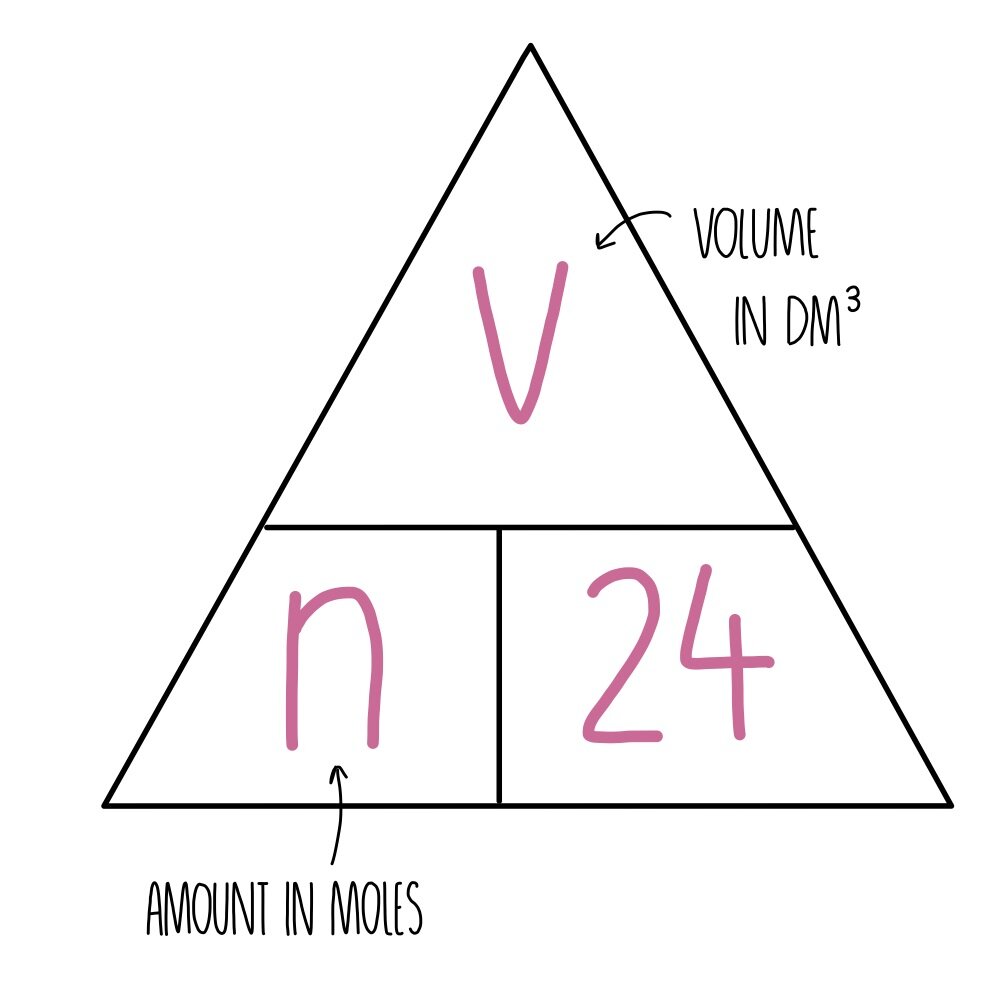
What Does A Mole Mean In Chemistry . the mole is the unit of measurement in the international system of units (si) for amount of substance. distinguish theoretical yield from actual yield. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Learn how to use the mole to convert between atoms, molecules, and. Learn how to use moles in. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Moles are a convenient unit used in chemistry to convert between amounts of. a mole is an si base unit for quantity that describes the number of particles. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities.
from www.thesciencehive.co.uk
Learn how to use the mole to convert between atoms, molecules, and. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. distinguish theoretical yield from actual yield. a mole is an si base unit for quantity that describes the number of particles. Moles are a convenient unit used in chemistry to convert between amounts of. the mole is the unit of measurement in the international system of units (si) for amount of substance. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use moles in. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities.
Amount of Substance* — the science sauce
What Does A Mole Mean In Chemistry Learn how to use the mole to convert between atoms, molecules, and. a mole is an si base unit for quantity that describes the number of particles. distinguish theoretical yield from actual yield. Moles are a convenient unit used in chemistry to convert between amounts of. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Learn how to use moles in. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use the mole to convert between atoms, molecules, and. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. the mole is the unit of measurement in the international system of units (si) for amount of substance.
From www.youtube.com
What is a mole? Definition and examples YouTube What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. distinguish theoretical yield from actual yield. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use the mole to convert between atoms, molecules, and. Learn how to. What Does A Mole Mean In Chemistry.
From www.pinterest.es
Repost compoundchem ・・・ 👨🔬 Here’s a quick explainer on the mole and What Does A Mole Mean In Chemistry a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Moles are a convenient unit used in chemistry to convert between amounts of. a mole is an si base unit for quantity that describes the number of particles. distinguish theoretical yield from actual yield. . What Does A Mole Mean In Chemistry.
From syatillakmk.blogspot.com
SimplyChemistry C3 MOLEMASSNO.PARTICLE CONVERSIONS What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. Moles are a convenient unit used in chemistry to convert between amounts of. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. distinguish theoretical yield from actual yield. a mole is the. What Does A Mole Mean In Chemistry.
From www.pinterest.com
Pin on Science Chemistry What Does A Mole Mean In Chemistry a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Moles are a convenient unit used in chemistry to convert between amounts of. Learn how to use the mole to convert between atoms, molecules, and. Learn how to use moles in. distinguish theoretical yield from actual. What Does A Mole Mean In Chemistry.
From quizlet.com
Chem 08 Reactions & Moles Diagram Quizlet What Does A Mole Mean In Chemistry Learn how to use moles in. distinguish theoretical yield from actual yield. the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is an si base unit for quantity that describes the number of particles. a mole is the si unit for the amount of a. What Does A Mole Mean In Chemistry.
From telgurus.co.uk
How to calculate moles in chemistry? Chemistry questionnaire What Does A Mole Mean In Chemistry Learn how to use moles in. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Learn how to use the mole to convert between atoms, molecules, and. Moles are a convenient unit used in chemistry to convert between amounts of. distinguish theoretical yield from actual. What Does A Mole Mean In Chemistry.
From www.slideserve.com
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061 What Does A Mole Mean In Chemistry mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use moles in. a mole is an si base unit for quantity that describes the number of particles. distinguish theoretical yield from actual yield. a mole is the si unit for the amount of a. What Does A Mole Mean In Chemistry.
From chemistry.stackexchange.com
What exactly is a mole? Chemistry Stack Exchange What Does A Mole Mean In Chemistry Moles are a convenient unit used in chemistry to convert between amounts of. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. distinguish theoretical yield from actual yield. Learn how to use the mole to convert between atoms, molecules, and. Learn how to use moles. What Does A Mole Mean In Chemistry.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use moles in. Learn how to use the mole to convert between atoms, molecules, and. the mole is. What Does A Mole Mean In Chemistry.
From www.pinterest.com
What does a Mole Mean? Teaching chemistry, High school chemistry What Does A Mole Mean In Chemistry Learn how to use moles in. Learn how to use the mole to convert between atoms, molecules, and. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities.. What Does A Mole Mean In Chemistry.
From afterskool.edu.sg
'O' Level Chemistry 101 Mole Concept Summary Guide — AfterSkool What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the. What Does A Mole Mean In Chemistry.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube What Does A Mole Mean In Chemistry Learn how to use the mole to convert between atoms, molecules, and. Learn how to use moles in. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. the mole is the unit of measurement in the international system of units (si) for amount of substance.. What Does A Mole Mean In Chemistry.
From www.shodor.org
Chemical Stoichiometry What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Moles are. What Does A Mole Mean In Chemistry.
From www.biologyexams4u.com
Normality, Molality, Molarity, Mole fraction, Percentage Solution What Does A Mole Mean In Chemistry mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is an si base unit for quantity that describes the number of particles. Learn how to use the mole to convert between atoms, molecules, and. a mole is the si unit for the amount of a substance,. What Does A Mole Mean In Chemistry.
From sciencenotes.org
Mole Ratio Definition and Examples What Does A Mole Mean In Chemistry Learn how to use moles in. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Moles are a convenient unit used in chemistry to convert between amounts of. Learn how to use the mole to convert between atoms, molecules, and. a mole is defined as a chemical unit, defined. What Does A Mole Mean In Chemistry.
From www.showme.com
Mole calculations CHEM 1 Science, Chemistry, Moles ShowMe What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. distinguish theoretical yield from actual yield. Moles are a convenient unit used in chemistry to convert between amounts of. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use the. What Does A Mole Mean In Chemistry.
From sciencenotes.org
What Is a Mole In Chemistry? Definition What Does A Mole Mean In Chemistry a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. the mole is the unit of measurement in the international system of units (si) for amount of substance. Moles are a convenient unit used in chemistry to convert between amounts of. Learn how to use the. What Does A Mole Mean In Chemistry.
From www.templateroller.com
Chemistry Cheat Sheet Mole Conversion Chart Download Printable PDF What Does A Mole Mean In Chemistry distinguish theoretical yield from actual yield. Learn how to use the mole to convert between atoms, molecules, and. Learn how to use moles in. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. mole, in chemistry, a standard scientific unit for measuring large quantities. What Does A Mole Mean In Chemistry.
From revisechemistry.uk
Chemical Calculations Edexcel T1 revisechemistry.uk What Does A Mole Mean In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use the mole to convert between atoms, molecules, and. a mole is an si base unit for quantity. What Does A Mole Mean In Chemistry.
From courses.lumenlearning.com
7.1 The Mole Concept Introductory Chemistry What Does A Mole Mean In Chemistry a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. distinguish theoretical yield from actual yield. the mole is the unit of measurement in the international system of units (si) for amount of substance. Learn how to use moles in. a mole is an. What Does A Mole Mean In Chemistry.
From www.docbrown.info
definition mole explained molar mass mol mols calculations how to read What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. a. What Does A Mole Mean In Chemistry.
From www.youtube.com
Stoichiometry Mole to Mole Conversions Molar Ratio Practice Problems What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Moles are a convenient unit used in chemistry to convert between amounts of. Learn how to use the. What Does A Mole Mean In Chemistry.
From za.pinterest.com
What does a Mole Mean? Chemistry worksheets, Teaching science, High What Does A Mole Mean In Chemistry mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. Moles are a convenient unit used in chemistry to convert between amounts of. the mole is the. What Does A Mole Mean In Chemistry.
From www.youtube.com
How To Convert Between Moles, Atoms, and Grams In Chemistry QUICK What Does A Mole Mean In Chemistry Moles are a convenient unit used in chemistry to convert between amounts of. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Learn how to use moles in. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the. What Does A Mole Mean In Chemistry.
From www.showme.com
Moles to atoms Chemistry ShowMe What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. a mole is an si base unit for quantity that describes the number of particles. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. the. What Does A Mole Mean In Chemistry.
From www.slideserve.com
PPT The Chemistry Mole PowerPoint Presentation, free download ID What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Learn how to use the mole to convert between atoms, molecules, and. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. mole, in chemistry, a standard. What Does A Mole Mean In Chemistry.
From surfguppy.com
The Mole relationship to Carbon Surfguppy Chemistry made easy What Does A Mole Mean In Chemistry distinguish theoretical yield from actual yield. Learn how to use moles in. Learn how to use the mole to convert between atoms, molecules, and. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. a mole is an si base unit for quantity that describes the number of particles.. What Does A Mole Mean In Chemistry.
From mungfali.com
The Mole Chemistry What Does A Mole Mean In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. Learn how to use moles in. a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. a mole is defined as a chemical unit, defined to be. What Does A Mole Mean In Chemistry.
From sciencenotes.org
Mole Fraction Formula and Calculation What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. Learn how to use the mole to convert between atoms, molecules, and. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. Learn how to use moles in. distinguish theoretical yield from actual yield.. What Does A Mole Mean In Chemistry.
From www.thesciencehive.co.uk
Amount of Substance* — the science sauce What Does A Mole Mean In Chemistry the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Moles are a convenient unit used in chemistry to convert between amounts of. a mole is the si unit for the. What Does A Mole Mean In Chemistry.
From www.slideserve.com
PPT The mole PowerPoint Presentation, free download ID1836804 What Does A Mole Mean In Chemistry Learn how to use the mole to convert between atoms, molecules, and. a mole is an si base unit for quantity that describes the number of particles. distinguish theoretical yield from actual yield. the mole is the unit of measurement in the international system of units (si) for amount of substance. Moles are a convenient unit used. What Does A Mole Mean In Chemistry.
From www.pinterest.co.uk
What does a mole look like? Teaching chemistry, Chemistry classroom What Does A Mole Mean In Chemistry Moles are a convenient unit used in chemistry to convert between amounts of. a mole is an si base unit for quantity that describes the number of particles. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. a mole is defined as a chemical unit, defined to be. What Does A Mole Mean In Chemistry.
From www.pinterest.com
What does a Mole Mean? Chemistry classroom, Chemistry teacher high What Does A Mole Mean In Chemistry a mole is an si base unit for quantity that describes the number of particles. mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms,. the mole is the unit of measurement in the international system of units (si) for amount of substance. a mole is the si. What Does A Mole Mean In Chemistry.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions What Does A Mole Mean In Chemistry a mole is the si unit for the amount of a substance, equal to 6.023 x 10 23 particles of the substance. the mole is the unit of measurement in the international system of units (si) for amount of substance. distinguish theoretical yield from actual yield. Learn how to use the mole to convert between atoms, molecules,. What Does A Mole Mean In Chemistry.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube What Does A Mole Mean In Chemistry a mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. distinguish theoretical yield from actual yield. the mole is the unit of measurement in the international system of units (si) for amount of substance. Learn how to use the mole to convert between atoms, molecules, and. Learn how to. What Does A Mole Mean In Chemistry.