Pvc Chemistry Structure . The chemical structure of the vinyl chloride repeating units is: Rigid pvc is easily machined, heat formed, welded, and. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. See the chemical formula and structure of polyvinyl.
from molecor.com
Rigid pvc is easily machined, heat formed, welded, and. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. See the chemical formula and structure of polyvinyl. The chemical structure of the vinyl chloride repeating units is: In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive.
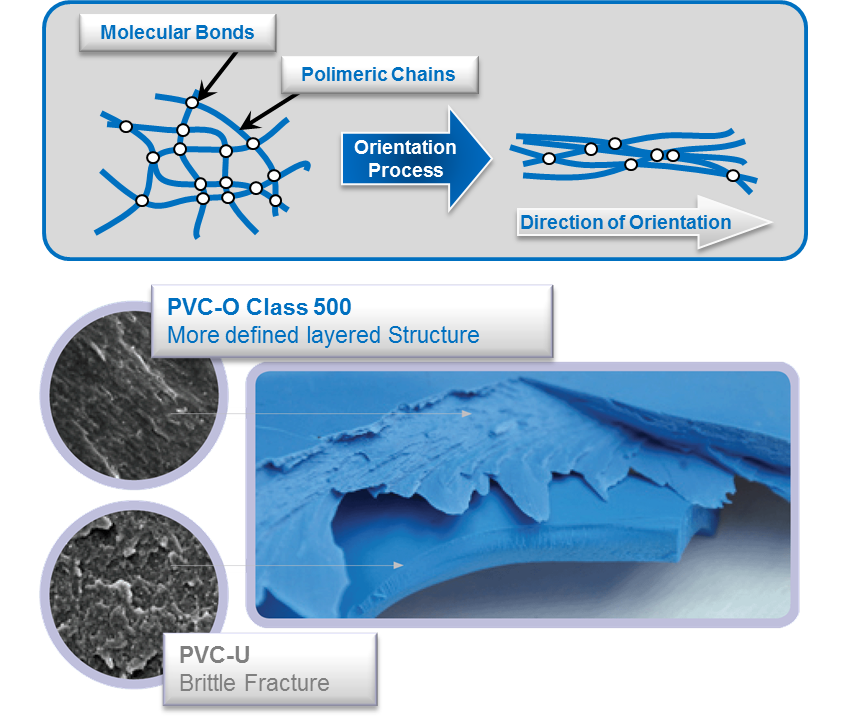
What is Molecularly Oriented PVC? Molecor
Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Rigid pvc is easily machined, heat formed, welded, and. See the chemical formula and structure of polyvinyl. The chemical structure of the vinyl chloride repeating units is: In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,.
From madisongroup.com
PVC Part 1 It's All About Composition The Madison Group Pvc Chemistry Structure The chemical structure of the vinyl chloride repeating units is: In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was. Pvc Chemistry Structure.
From pixels.com
Polycarbonate Plastic Chemical Structure 4 Photograph by Molekuul Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. The chemical structure of the vinyl chloride repeating units is: Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Rigid pvc is easily machined, heat formed, welded, and. See the chemical formula and structure of polyvinyl. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. See the chemical formula and structure of polyvinyl. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Learn about polyvinyl chloride,. Pvc Chemistry Structure.
From www.dreamstime.com
Poly(vinyl Chloride) Plastic (PVC), Chemical Structure. Used in Pvc Chemistry Structure The chemical structure of the vinyl chloride repeating units is: In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. See the chemical formula and structure of polyvinyl. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872,. Pvc Chemistry Structure.
From www.vectorstock.com
Vinyl chloride pvc chemical structure Royalty Free Vector Pvc Chemistry Structure In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. See the chemical formula and structure of polyvinyl. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french. Pvc Chemistry Structure.
From www.alamy.com
Pvc polymer, molecular model hires stock photography and images Alamy Pvc Chemistry Structure In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Pvc was prepared by the french chemist henri victor regnault in 1835. Pvc Chemistry Structure.
From www.sciencephoto.com
PVC polymer, molecular model Stock Image C009/8149 Science Photo Pvc Chemistry Structure In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented. Pvc Chemistry Structure.
From www.pinterest.com
Polyvinyl chloride (PVC) and vinyl chloride monomer molecule Pvc Chemistry Structure Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. See the chemical formula and structure of polyvinyl. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Rigid pvc is easily machined, heat formed, welded, and. Pvc was. Pvc Chemistry Structure.
From mavink.com
Plastic Atomic Structure Pvc Chemistry Structure See the chemical formula and structure of polyvinyl. The chemical structure of the vinyl chloride repeating units is: Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Pvc was prepared by the french chemist henri victor regnault in 1835 and then. Pvc Chemistry Structure.
From civilboost360.blogspot.com
What is PVC ? What are the advantages of it as a construction material Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. The chemical structure of the vinyl chloride repeating units is: In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Pvc is a polymer,. Pvc Chemistry Structure.
From read.nxtbook.com
Plastics It’s All About Molecular Structure Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. See the chemical formula and structure of polyvinyl. The chemical structure of the vinyl chloride repeating units is: Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Rigid. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. The chemical structure of the vinyl chloride repeating units is: Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Pvc was. Pvc Chemistry Structure.
From www.scribd.com
PVC Chemistry PDF Polyvinyl Chloride Polymerization Pvc Chemistry Structure Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Learn about polyvinyl. Pvc Chemistry Structure.
From www.iqsdirectory.com
Polyvinyl Chloride (PVC) Types of Tubing, Uses & Benefits Pvc Chemistry Structure See the chemical formula and structure of polyvinyl. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Learn about polyvinyl chloride, its characteristics, how it. Pvc Chemistry Structure.
From www.dreamstime.com
Chemical Formula of PVC with Black Pen Stock Photo Image of structure Pvc Chemistry Structure See the chemical formula and structure of polyvinyl. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Rigid pvc is easily machined, heat formed, welded, and. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization. Pvc Chemistry Structure.
From www.dreamstime.com
Polyvinyl Chloride Plastic PVC, Chemical Structure. Used in Production Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. In this chapter,. Pvc Chemistry Structure.
From molecor.com
What is Molecularly Oriented PVC? Molecor Pvc Chemistry Structure See the chemical formula and structure of polyvinyl. Rigid pvc is easily machined, heat formed, welded, and. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis. Pvc Chemistry Structure.
From www.dreamstime.com
Polyvinyl Chloride Plastic PVC, Chemical Structure. Used in Production Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. See the chemical formula and structure of polyvinyl. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. See the chemical formula and structure of polyvinyl. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Rigid pvc is easily. Pvc Chemistry Structure.
From www.dreamstime.com
Polyethylene PE, Polythene, Polyethene Plastic, Chemical Structure Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. The chemical structure of the vinyl chloride repeating units is: Rigid pvc is easily machined, heat formed, welded, and. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. See the chemical formula and structure of polyvinyl. Polyvinyl chloride (pvc) is a. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Rigid pvc is easily machined, heat formed, welded, and. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. The chemical structure of the vinyl chloride. Pvc Chemistry Structure.
From read.nxtbook.com
Plastics It’s All About Molecular Structure Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french chemist henri victor regnault in 1835 and then. Pvc Chemistry Structure.
From www.shutterstock.com
Structural Chemical Formula And Model Of Polyvinyl Chloride Molecule Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Rigid pvc is easily machined, heat formed, welded, and. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist. Pvc Chemistry Structure.
From www.alamy.com
poly(vinyl chloride) plastic (PVC), chemical structure. Used in Pvc Chemistry Structure In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Rigid pvc is easily machined, heat formed, welded, and. The chemical structure of the vinyl chloride repeating units is: Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc was prepared by the french chemist henri. Pvc Chemistry Structure.
From www.mdpi.com
Polymers Free FullText Cardanol Groups Grafted on Poly(vinyl Pvc Chemistry Structure Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Polyvinyl chloride (pvc). Pvc Chemistry Structure.
From www.vectorstock.com
Vinyl chloride pvc chemical structure Royalty Free Vector Pvc Chemistry Structure Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Learn. Pvc Chemistry Structure.
From www.sinodoschemistry.com
SinodosChemistry Polymers classification Pvc Chemistry Structure Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. The chemical structure of the vinyl chloride repeating units is: Rigid pvc is easily machined, heat formed, welded, and. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate the polymerization of. Rigid pvc is easily machined, heat formed, welded, and. Pvc is a polymer, a large molecule. Pvc Chemistry Structure.
From www.alamy.com
Polycarbonate (PC) plastic, chemical structure. Made from phosgene Pvc Chemistry Structure The chemical structure of the vinyl chloride repeating units is: Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Rigid pvc is easily machined, heat formed, welded, and.. Pvc Chemistry Structure.
From exojucsti.blob.core.windows.net
Pvc Monomer Structure at John Fleming blog Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented. Pvc Chemistry Structure.
From www.alamy.com
Polyvinyl chloride pvc molecule hires stock photography and images Alamy Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. Rigid pvc is easily machined, heat formed, welded, and. See the chemical formula and structure of polyvinyl. Pvc is a polymer, a large molecule made from repeating units linked by covalent bonds. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc was. Pvc Chemistry Structure.
From www.youtube.com
maxresdefault.jpg Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. The chemical structure of the vinyl chloride repeating units is: Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. In this chapter, detailed description of pvc including brief history, chemical structure, laboratory scale synthesis and polymerization processes like radical,. See the chemical formula. Pvc Chemistry Structure.
From polyacs.org
poly(vinyl chloride) plastic (PVC), chemical structure linear Pvc Chemistry Structure The chemical structure of the vinyl chloride repeating units is: Rigid pvc is easily machined, heat formed, welded, and. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not patented until 1912, when another german chemist, friedrich heinrich august klatte, used sunlight to initiate. Pvc Chemistry Structure.
From preparatorychemistry.com
Addition Polymers Pvc Chemistry Structure Learn about polyvinyl chloride, its characteristics, how it is made, and its uses. See the chemical formula and structure of polyvinyl. Polyvinyl chloride (pvc) is a flexible or rigid material that is chemically nonreactive. Pvc was prepared by the french chemist henri victor regnault in 1835 and then by the german chemist eugen baumann in 1872, but it was not. Pvc Chemistry Structure.