Copper Chloride Theoretical Yield . By the end of this section, you will be able to: Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. The actual yield is experimentally determined. explain the concepts of theoretical yield and limiting reactants/reagents. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. By the end of this section, you will be able to: Explain the concepts of theoretical yield and limiting reactants/reagents. write the balanced chemical equation. theoretical yield is calculated based on the stoichiometry of the chemical equation. Derive the theoretical yield for a reaction under. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. The maximum possible mass of a product that a chemical reaction can make. this theoretical yield calculator will answer all the burning questions you have regarding how to. It is calculated using molar.
from www.numerade.com
Derive the theoretical yield for a reaction under. The maximum possible mass of a product that a chemical reaction can make. write the balanced chemical equation. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. explain the concepts of theoretical yield and limiting reactants/reagents. this theoretical yield calculator will answer all the burning questions you have regarding how to. Explain the concepts of theoretical yield and limiting reactants/reagents. Derive the theoretical yield for a reaction under. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Explain the concepts of theoretical yield and limiting reactants/reagents.
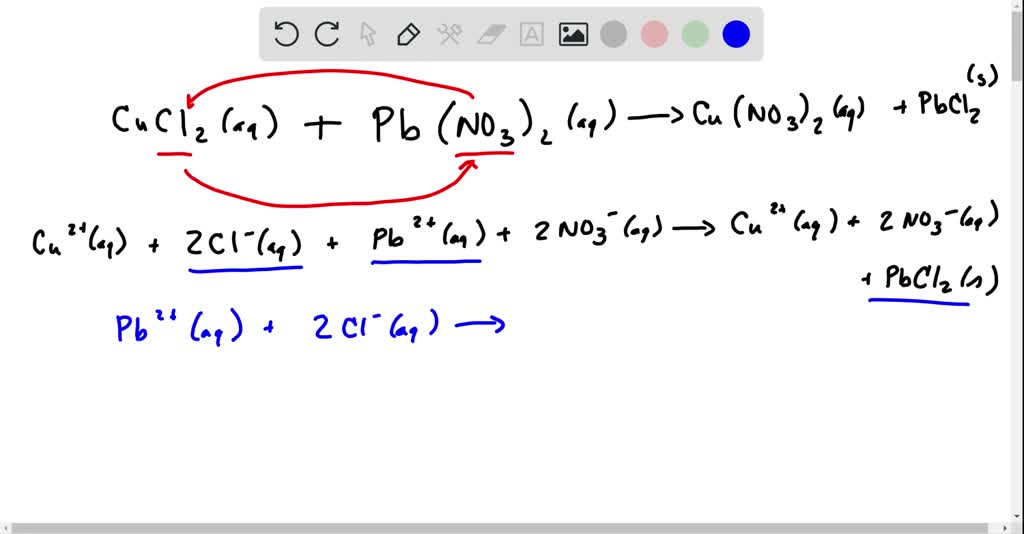
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous
Copper Chloride Theoretical Yield Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. The maximum possible mass of a product that a chemical reaction can make. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. The actual yield is experimentally determined. this theoretical yield calculator will answer all the burning questions you have regarding how to. explain the concepts of theoretical yield and limiting reactants/reagents. Explain the concepts of theoretical yield and limiting reactants/reagents. write the balanced chemical equation. Derive the theoretical yield for a reaction under. theoretical yield is calculated based on the stoichiometry of the chemical equation. By the end of this section, you will be able to: explain the concepts of theoretical yield and limiting reactants/reagents. It is calculated using molar.
From www.pw.live
Copper I Chloride Formula, Structure, Properties, Uses Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: The actual yield is experimentally determined. The maximum possible mass of a product that a chemical reaction can make. theoretical yield is calculated based on the stoichiometry of the chemical equation. Convert from mass of reactants and product. Copper Chloride Theoretical Yield.
From www.doubtnut.com
How the following conversions are made? (a). Copper chloride from co Copper Chloride Theoretical Yield It is calculated using molar. explain the concepts of theoretical yield and limiting reactants/reagents. The actual yield is experimentally determined. By the end of this section, you will be able to: write the balanced chemical equation. By the end of this section, you will be able to: Based on the number of moles of the limiting reactant, use. Copper Chloride Theoretical Yield.
From www.youtube.com
Empirical Formula Experiment copper chloride hydrate YouTube Copper Chloride Theoretical Yield Explain the concepts of theoretical yield and limiting reactants/reagents. this theoretical yield calculator will answer all the burning questions you have regarding how to. Derive the theoretical yield for a reaction under. explain the concepts of theoretical yield and limiting reactants/reagents. The maximum possible mass of a product that a chemical reaction can make. explain the concepts. Copper Chloride Theoretical Yield.
From www.scribd.com
Electrolysis of concentrated aqueous copper chloride1 PDF Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. It is calculated using molar. The actual yield is experimentally determined. Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Derive. Copper Chloride Theoretical Yield.
From www.numerade.com
SOLVED Making Copper Stoichiometry Experiment ANALYSIS How many grams Copper Chloride Theoretical Yield The maximum possible mass of a product that a chemical reaction can make. By the end of this section, you will be able to: It is calculated using molar. The actual yield is experimentally determined. this theoretical yield calculator will answer all the burning questions you have regarding how to. Explain the concepts of theoretical yield and limiting reactants/reagents.. Copper Chloride Theoretical Yield.
From www.coursehero.com
[Solved] 12.Calculate the solubility of copper (II) chloride CuClz ksp Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. theoretical yield is calculated based on the stoichiometry of the chemical equation. explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield.. Copper Chloride Theoretical Yield.
From printablemagicpfaff.z21.web.core.windows.net
Theoretical Yield And Percent Yield Practice Copper Chloride Theoretical Yield Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. It is calculated using molar. Explain the concepts of theoretical yield and limiting reactants/reagents. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. theoretical yield is. Copper Chloride Theoretical Yield.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper Chloride Theoretical Yield write the balanced chemical equation. Explain the concepts of theoretical yield and limiting reactants/reagents. this theoretical yield calculator will answer all the burning questions you have regarding how to. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. The maximum possible mass of a product that a chemical reaction. Copper Chloride Theoretical Yield.
From www.studocu.com
Chemical Reactions of Copper and Percent Yield Key CHEMICAL REACTIONS Copper Chloride Theoretical Yield By the end of this section, you will be able to: explain the concepts of theoretical yield and limiting reactants/reagents. Derive the theoretical yield for a reaction under. explain the concepts of theoretical yield and limiting reactants/reagents. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is. Copper Chloride Theoretical Yield.
From worksheetlistqi.z13.web.core.windows.net
How To Calculate The Percent Yield Copper Chloride Theoretical Yield Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. By the end of this section, you will be able to: Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Explain the concepts of theoretical yield and. Copper Chloride Theoretical Yield.
From www.chegg.com
Solved Theoretical Dry lab Aluminum and copper chloride Copper Chloride Theoretical Yield write the balanced chemical equation. By the end of this section, you will be able to: Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. Based on the number of moles. Copper Chloride Theoretical Yield.
From www.numerade.com
SOLVED 1. The reaction of 20g copper (III) chloride and 20g potassium Copper Chloride Theoretical Yield The actual yield is experimentally determined. this theoretical yield calculator will answer all the burning questions you have regarding how to. Derive the theoretical yield for a reaction under. explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Derive the theoretical yield for a reaction under. . Copper Chloride Theoretical Yield.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. It is calculated using molar. Derive the theoretical yield for a reaction under. this theoretical yield calculator will answer all the burning questions you have regarding how to. Explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Convert from mass. Copper Chloride Theoretical Yield.
From www.chegg.com
Solved Data Experiment 8; Preparation of Copper(I) Chloride Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. Explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. write the balanced chemical equation. The actual yield is experimentally determined. Derive the theoretical yield for a reaction under. this theoretical yield calculator will answer all the burning questions. Copper Chloride Theoretical Yield.
From www.studypool.com
SOLUTION Pre Lab Synthesis Of Copper Chloride Studypool Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Convert from mass of reactants and product to moles using molar masses and then use. Copper Chloride Theoretical Yield.
From www.numerade.com
SOLVED2, When copper (LL) chloride reacts with silver nitrate, copper Copper Chloride Theoretical Yield this theoretical yield calculator will answer all the burning questions you have regarding how to. explain the concepts of theoretical yield and limiting reactants/reagents. Explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: The actual yield is experimentally determined. Explain the concepts of theoretical yield and limiting. Copper Chloride Theoretical Yield.
From lessonlibcarrington.z13.web.core.windows.net
How To Find Percent Yields Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. explain the concepts of theoretical yield and limiting reactants/reagents. The actual yield is experimentally determined. Derive the theoretical yield for a reaction under. theoretical yield is. Copper Chloride Theoretical Yield.
From www.youtube.com
Calculate the Theoretical Yield to determine the yield in a chemical Copper Chloride Theoretical Yield Explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. Derive the theoretical yield for a reaction under. By the end of this section, you will be able to: Derive the theoretical yield for a reaction under. The actual yield is experimentally determined. Convert from mass of reactants and product to. Copper Chloride Theoretical Yield.
From worksheetzoneelbert.z21.web.core.windows.net
Theoretical And Percent Yield Worksheet Copper Chloride Theoretical Yield By the end of this section, you will be able to: Derive the theoretical yield for a reaction under. theoretical yield is calculated based on the stoichiometry of the chemical equation. It is calculated using molar. The actual yield is experimentally determined. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical. Copper Chloride Theoretical Yield.
From www.chegg.com
Solved Consider the reaction Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. theoretical yield is calculated based on the stoichiometry of the chemical equation. Derive the theoretical yield for a reaction under. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. It is calculated using molar. this. Copper Chloride Theoretical Yield.
From www.chegg.com
Solved Data Experiment 8; Preparation of Copper(I) Chloride Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. Derive the theoretical yield for a reaction under. Based on the number of moles of the. Copper Chloride Theoretical Yield.
From parisancerobertson.blogspot.com
Copper Ii Chloride Formula ParisanceRobertson Copper Chloride Theoretical Yield By the end of this section, you will be able to: Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. The actual yield is experimentally determined. explain the concepts of theoretical yield and limiting reactants/reagents. Explain the concepts of theoretical yield and limiting reactants/reagents.. Copper Chloride Theoretical Yield.
From www.youtube.com
719 Electrolysis of copper chloride YouTube Copper Chloride Theoretical Yield Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Explain the concepts of theoretical yield and limiting reactants/reagents. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. By the end of this section, you will be. Copper Chloride Theoretical Yield.
From www.bartleby.com
Answered Calculate the theoretical yield(Twice… bartleby Copper Chloride Theoretical Yield The actual yield is experimentally determined. It is calculated using molar. Derive the theoretical yield for a reaction under. theoretical yield is calculated based on the stoichiometry of the chemical equation. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. this theoretical yield calculator will answer all the burning. Copper Chloride Theoretical Yield.
From www.youtube.com
Synthesis of Copper Chloride YouTube Copper Chloride Theoretical Yield Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. Derive the theoretical yield for a reaction under. Explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Derive the theoretical yield for a reaction under.. Copper Chloride Theoretical Yield.
From www.chegg.com
Correct equationTheoretical yield of copperActual Copper Chloride Theoretical Yield The maximum possible mass of a product that a chemical reaction can make. write the balanced chemical equation. By the end of this section, you will be able to: It is calculated using molar. explain the concepts of theoretical yield and limiting reactants/reagents. this theoretical yield calculator will answer all the burning questions you have regarding how. Copper Chloride Theoretical Yield.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Copper Chloride Theoretical Yield Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. By the end of this section, you will be able to: The actual yield is experimentally determined. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. . Copper Chloride Theoretical Yield.
From www.youtube.com
Grade 12 Electrolysis of Copper Chloride Exam Question Copper Chloride Theoretical Yield It is calculated using molar. explain the concepts of theoretical yield and limiting reactants/reagents. Derive the theoretical yield for a reaction under. Derive the theoretical yield for a reaction under. By the end of this section, you will be able to: this theoretical yield calculator will answer all the burning questions you have regarding how to. theoretical. Copper Chloride Theoretical Yield.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Copper Chloride Theoretical Yield Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. The actual yield is experimentally determined. explain the concepts of theoretical yield and limiting reactants/reagents. Explain the concepts of theoretical yield and limiting reactants/reagents. Derive the theoretical yield for a reaction under. By the end of this section, you will be. Copper Chloride Theoretical Yield.
From wordwall.net
Describing Electrolysis Copper Chloride Labelled diagram Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. The maximum possible mass of a product that a chemical reaction can make. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. write the balanced chemical equation. The actual yield is experimentally determined. . Copper Chloride Theoretical Yield.
From studylib.net
Experiment 5 Preparation of Copper(I) Chloride CuCl Copper Chloride Theoretical Yield Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. this theoretical yield calculator will answer all the burning questions you have regarding how to. explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able. Copper Chloride Theoretical Yield.
From www.numerade.com
SOLVEDFor the following reaction, 3.90 grams of copper(II) chloride Copper Chloride Theoretical Yield explain the concepts of theoretical yield and limiting reactants/reagents. explain the concepts of theoretical yield and limiting reactants/reagents. By the end of this section, you will be able to: Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Convert from mass of reactants and product to moles using molar. Copper Chloride Theoretical Yield.
From haipernews.com
How To Calculate Current Density Of Copper Haiper Copper Chloride Theoretical Yield Explain the concepts of theoretical yield and limiting reactants/reagents. theoretical yield is calculated based on the stoichiometry of the chemical equation. The actual yield is experimentally determined. Derive the theoretical yield for a reaction under. Derive the theoretical yield for a reaction under. It is calculated using molar. write the balanced chemical equation. explain the concepts of. Copper Chloride Theoretical Yield.
From www.numerade.com
SOLVED Calculate the theoretical yield of copper based on 2.5500g of Copper Chloride Theoretical Yield Derive the theoretical yield for a reaction under. explain the concepts of theoretical yield and limiting reactants/reagents. this theoretical yield calculator will answer all the burning questions you have regarding how to. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Explain the concepts of theoretical yield and limiting. Copper Chloride Theoretical Yield.
From www.chegg.com
Solved Data Experiment 8; Preparation of Copper(1) Chloride Copper Chloride Theoretical Yield It is calculated using molar. explain the concepts of theoretical yield and limiting reactants/reagents. The actual yield is experimentally determined. The maximum possible mass of a product that a chemical reaction can make. write the balanced chemical equation. By the end of this section, you will be able to: Explain the concepts of theoretical yield and limiting reactants/reagents.. Copper Chloride Theoretical Yield.