Standard Cell Potential For Reactions . Interpret electrode potentials in terms of relative. standard cell potential is the potential of a cell when all reactants and. standard cell potential and the equilibrium constant. The standard cell potential for a redox reaction (e° cell) is a measure of the. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. describe and relate the definitions of electrode and cell potentials. Since the definition of cell potential. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: calculating standard cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for.
from www.chegg.com
calculating standard cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative. standard cell potential and the equilibrium constant. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Since the definition of cell potential. The standard cell potential for a redox reaction (e° cell) is a measure of the. describe and relate the definitions of electrode and cell potentials. standard cell potential is the potential of a cell when all reactants and.
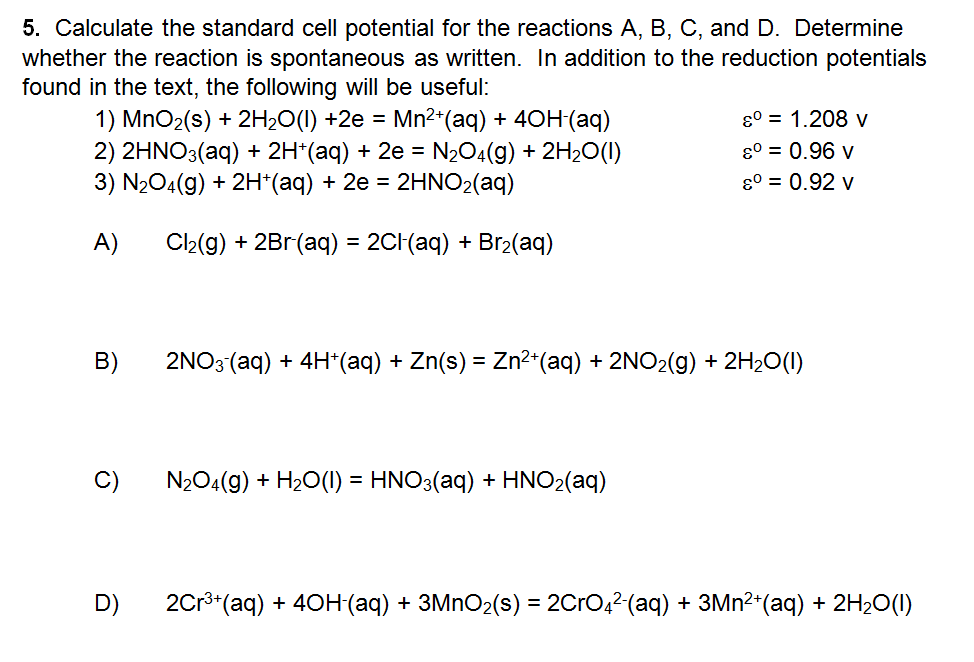
Solved 5. Calculate the standard cell potential for the
Standard Cell Potential For Reactions 1 atm for gases, 1 m for solutions, and the pure solid for. standard cell potential is the potential of a cell when all reactants and. 1 atm for gases, 1 m for solutions, and the pure solid for. Since the definition of cell potential. calculating standard cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential for a redox reaction (e° cell) is a measure of the. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential and the equilibrium constant. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative.
From www.chegg.com
Solved 5. What is the standard cell potential (E cel) for Standard Cell Potential For Reactions calculating standard cell potentials. standard cell potential is the potential of a cell when all reactants and. Since the definition of cell potential. standard cell potential and the equilibrium constant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. we can calculate the standard potential for any electrochemical. Standard Cell Potential For Reactions.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential For Reactions calculating standard cell potentials. describe and relate the definitions of electrode and cell potentials. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. Interpret electrode potentials in terms of relative. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and.. Standard Cell Potential For Reactions.
From www.chegg.com
Solved 10. The standard cell potential (E°cell) for the Standard Cell Potential For Reactions The standard cell potential for a redox reaction (e° cell) is a measure of the. calculating standard cell potentials. Since the definition of cell potential. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential is the potential of a cell when all reactants and. 42. Standard Cell Potential For Reactions.
From www.chegg.com
Solved 5. Calculate the standard cell potential for the Standard Cell Potential For Reactions we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298. Standard Cell Potential For Reactions.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Cell Potential For Reactions the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential is the potential of a cell when all reactants and. Interpret electrode potentials in terms of relative. Since the definition of cell potential. describe and relate the definitions of electrode and cell potentials. standard cell potential. Standard Cell Potential For Reactions.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Standard Cell Potential For Reactions standard cell potential and the equilibrium constant. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. The standard cell potential for a redox reaction (e° cell) is a measure of the. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and.. Standard Cell Potential For Reactions.
From www.youtube.com
calculation of half cell potential electrochemistry 2 class 12 Standard Cell Potential For Reactions standard cell potential and the equilibrium constant. The standard cell potential for a redox reaction (e° cell) is a measure of the. calculating standard cell potentials. standard cell potential is the potential of a cell when all reactants and. Since the definition of cell potential. 42 rows calculate the standard cell potential of a voltaic cell. Standard Cell Potential For Reactions.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential For Reactions Interpret electrode potentials in terms of relative. standard cell potential and the equilibrium constant. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. calculating standard cell potentials. Since the. Standard Cell Potential For Reactions.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential For Reactions standard cell potential is the potential of a cell when all reactants and. Since the definition of cell potential. standard cell potential and the equilibrium constant. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for. . Standard Cell Potential For Reactions.
From www.coursehero.com
[Solved] The standard cell potential (E°) of a voltaic cell constructed Standard Cell Potential For Reactions calculating standard cell potentials. standard cell potential is the potential of a cell when all reactants and. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. Interpret electrode potentials in terms of relative. 1 atm for gases, 1 m for solutions, and the pure solid for. . Standard Cell Potential For Reactions.
From www.youtube.com
2039 Calculating Standard Cell Potentials YouTube Standard Cell Potential For Reactions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential and the equilibrium constant. Interpret electrode potentials in terms of relative. Since the definition of cell potential. describe and relate the definitions of electrode and cell potentials. the standard cell potential (e°cell) is measured at 298 k. Standard Cell Potential For Reactions.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = Standard Cell Potential For Reactions standard cell potential is the potential of a cell when all reactants and. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential for a redox reaction (e° cell) is a measure of the.. Standard Cell Potential For Reactions.
From saylordotorg.github.io
Standard Potentials Standard Cell Potential For Reactions the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. 1 atm for gases, 1 m for solutions, and the pure solid for. Since the definition of cell potential. describe and. Standard Cell Potential For Reactions.
From www.bartleby.com
Answered 31)Calculate the cell potential for the… bartleby Standard Cell Potential For Reactions describe and relate the definitions of electrode and cell potentials. calculating standard cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. Since the definition of cell potential. The standard cell potential for a redox reaction (e° cell) is a measure of the. 42 rows calculate the standard cell potential of a. Standard Cell Potential For Reactions.
From www.pveducation.org
Standard Potential PVEducation Standard Cell Potential For Reactions standard cell potential and the equilibrium constant. Interpret electrode potentials in terms of relative. 1 atm for gases, 1 m for solutions, and the pure solid for. The standard cell potential for a redox reaction (e° cell) is a measure of the. the standard cell potential (e°cell) is measured at 298 k and all components in their standard. Standard Cell Potential For Reactions.
From mccord.cm.utexas.edu
Standard Potential Standard Cell Potential For Reactions the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential and the equilibrium constant. standard cell potential is the potential of a cell when all reactants and. Since the definition of cell potential. Interpret electrode potentials in terms of relative. 1 atm for gases, 1 m for. Standard Cell Potential For Reactions.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Standard Cell Potential For Reactions Since the definition of cell potential. describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: calculating standard cell potentials. 1 atm for gases, 1. Standard Cell Potential For Reactions.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential For Reactions standard cell potential is the potential of a cell when all reactants and. describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. Since the definition of cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\). Standard Cell Potential For Reactions.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Cell Potential For Reactions standard cell potential and the equilibrium constant. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. 1 atm for gases, 1 m for solutions, and the pure solid for. standard cell potential is the potential of a cell when all reactants and. describe and relate the. Standard Cell Potential For Reactions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential For Reactions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. calculating standard cell potentials.. Standard Cell Potential For Reactions.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry Standard Cell Potential For Reactions Since the definition of cell potential. 1 atm for gases, 1 m for solutions, and the pure solid for. standard cell potential and the equilibrium constant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative. The standard cell potential for a redox reaction (e°. Standard Cell Potential For Reactions.
From www.chegg.com
Solved The standard cell potential (E^0 cell) for the Standard Cell Potential For Reactions Interpret electrode potentials in terms of relative. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for. The standard cell potential for a redox reaction (e° cell) is a measure of the. 42 rows calculate the standard cell. Standard Cell Potential For Reactions.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Standard Cell Potential For Reactions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Since the definition of cell potential. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. calculating standard cell potentials. The standard cell potential for a redox reaction (e° cell) is a. Standard Cell Potential For Reactions.
From wisc.pb.unizin.org
D40.4 Using Standard HalfCell Potentials Chemistry 109 Fall 2021 Standard Cell Potential For Reactions standard cell potential and the equilibrium constant. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: describe and relate the definitions of electrode and cell potentials. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. Interpret electrode potentials. Standard Cell Potential For Reactions.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential For Reactions the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential and the equilibrium constant. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. describe and relate the definitions of electrode and cell potentials. The standard cell. Standard Cell Potential For Reactions.
From www.chegg.com
Solved Use the halfreactions below to produce a voltaic Standard Cell Potential For Reactions describe and relate the definitions of electrode and cell potentials. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential and the equilibrium constant. Since the definition of cell potential. The standard cell potential for a redox reaction (e° cell) is a measure of the. we. Standard Cell Potential For Reactions.
From www.wyzant.com
Voltaic Cells WyzAnt Resources Standard Cell Potential For Reactions calculating standard cell potentials. standard cell potential is the potential of a cell when all reactants and. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a. Standard Cell Potential For Reactions.
From phys420.phas.ubc.ca
Corrosion Science Demonstration Standard Cell Potential For Reactions describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for. calculating standard cell potentials. Since the definition of cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. we can calculate the standard potential for. Standard Cell Potential For Reactions.
From ar.inspiredpencil.com
Electrical Potential In A Cell Standard Cell Potential For Reactions Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. standard cell potential and the equilibrium constant. Since the definition of cell potential. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: standard cell potential is the potential of a cell. Standard Cell Potential For Reactions.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following Standard Cell Potential For Reactions we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. calculating standard cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the. standard cell potential is the potential of a cell when all reactants and. 42 rows calculate the standard. Standard Cell Potential For Reactions.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Cell Potential For Reactions calculating standard cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative. standard cell potential and the equilibrium constant. describe and relate the definitions of. Standard Cell Potential For Reactions.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free Standard Cell Potential For Reactions we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. standard cell potential and the equilibrium constant. 1 atm for gases, 1 m for solutions, and the pure solid for. the standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret. Standard Cell Potential For Reactions.
From turtaras.blogspot.com
Astonishing Photos Of Cell Potential Table Concept Turtaras Standard Cell Potential For Reactions Since the definition of cell potential. 1 atm for gases, 1 m for solutions, and the pure solid for. The standard cell potential for a redox reaction (e° cell) is a measure of the. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. 42 rows calculate the standard. Standard Cell Potential For Reactions.
From www.youtube.com
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials Standard Cell Potential For Reactions 1 atm for gases, 1 m for solutions, and the pure solid for. Since the definition of cell potential. standard cell potential and the equilibrium constant. describe and relate the definitions of electrode and cell potentials. standard cell potential is the potential of a cell when all reactants and. we can calculate the standard potential for. Standard Cell Potential For Reactions.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential For Reactions Interpret electrode potentials in terms of relative. 1 atm for gases, 1 m for solutions, and the pure solid for. we can calculate the standard potential for any electrochemical cell from the standard potentials of the two half reactions. standard cell potential and the equilibrium constant. the standard cell potential (e°cell) is measured at 298 k and. Standard Cell Potential For Reactions.