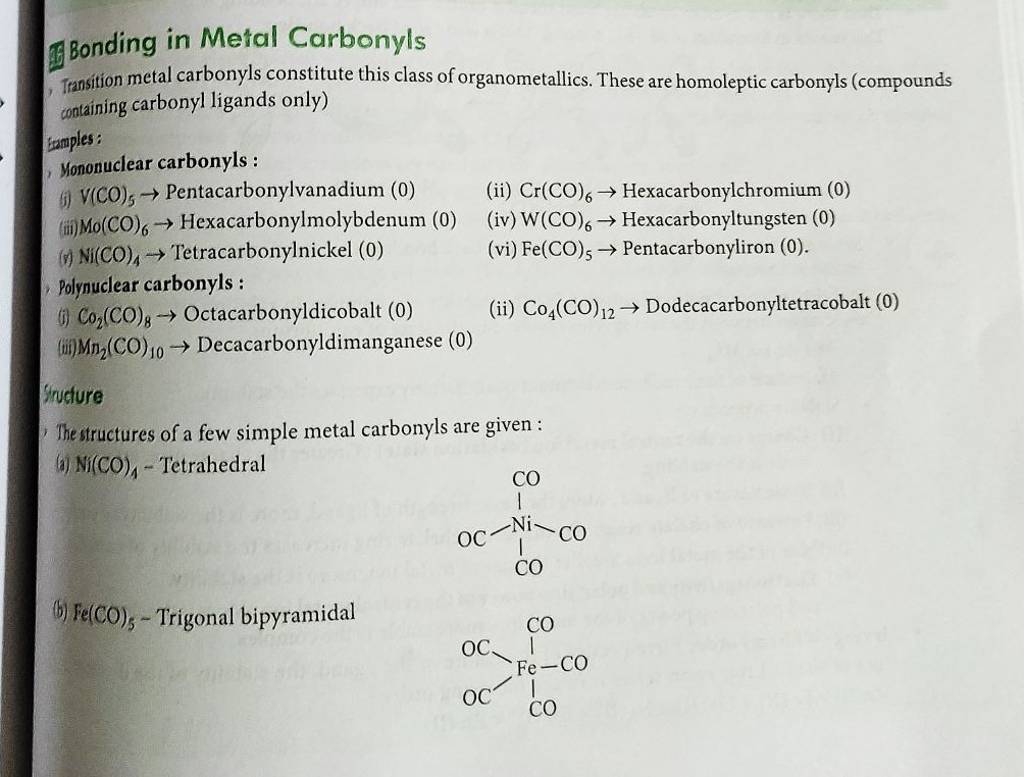
Bonding In Metal Carbonyls Class 12 Notes . Discuss the nature of bonding in metal carbonyls. (a) in coordination compounds, metals show two types of valencies : (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. (2) when co acts as a ligand, this orbital. ️📚👉 watch full free course videos: In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic.
from askfilo.com
(a) in coordination compounds, metals show two types of valencies : (2) when co acts as a ligand, this orbital. Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. ️📚👉 watch full free course videos: (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Discuss the nature of bonding in metal carbonyls.
I9 Bonding in Metal Carbonyls , Transition metal carbonyls constitute thi..
Bonding In Metal Carbonyls Class 12 Notes ️📚👉 watch full free course videos: The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (2) when co acts as a ligand, this orbital. ️📚👉 watch full free course videos: (a) in coordination compounds, metals show two types of valencies : In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. Discuss the nature of bonding in metal carbonyls. Know about the synergic effect and.
From www.youtube.com
Class 12 Chemistry Bonding in Metal Carbonyls in Chapter 9 Coordination Bonding In Metal Carbonyls Class 12 Notes In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. (2) when co acts as a ligand, this orbital. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (a) in coordination compounds, metals show two types of valencies : Discuss the nature of. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Class 12 XII Chemistry Coordination Chemistry Bonding in Metal Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: Know about the synergic effect and. (2) when co acts as a ligand, this orbital. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (1) in co molecule the. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Werner Theory and Bonding in Metal Carbonyls I Class 12th Chemistry Bonding In Metal Carbonyls Class 12 Notes ️📚👉 watch full free course videos: The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Know about the synergic effect and. (a) in coordination compounds, metals show two types of valencies : (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. (2) when co acts. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in Metal Carbonyls CoOrdination Compounds Chemistry Class Bonding In Metal Carbonyls Class 12 Notes In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Discuss the nature of bonding in metal carbonyls. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (a) in coordination compounds,. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Metal Carbonyls Application of Coordination Compounds Coordination Bonding In Metal Carbonyls Class 12 Notes ️📚👉 watch full free course videos: (a) in coordination compounds, metals show two types of valencies : (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Discuss the nature of. Bonding In Metal Carbonyls Class 12 Notes.
From www.doubtnut.com
Explain bonding in metal carbonyls. Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (2) when co acts as a ligand, this orbital. ️📚👉 watch full free course videos: Know about the synergic effect and. (1). Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Coordination Compounds 11 Bonding in Metal Carbonyls Class 12th Bonding In Metal Carbonyls Class 12 Notes (2) when co acts as a ligand, this orbital. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Know about the synergic effect and. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Discuss the nature of bonding in metal carbonyls. ️📚👉 watch full free course videos: (a). Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
bonding in metal carbonyls coordination and bio coordination compounds Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies : In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. (1) in co molecule the highest occupied molecular orbital is a. Bonding In Metal Carbonyls Class 12 Notes.
From askfilo.com
rganometallic compounds Synergic bonding in metal carbonyls Carbon monoxi.. Bonding In Metal Carbonyls Class 12 Notes The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Know about the synergic effect and. Discuss the nature of bonding in metal carbonyls. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: ️📚👉 watch full free course videos: (a) in coordination compounds, metals show two types of valencies. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Nature of bonding in Metal Carbonyls easy notes YouTube Bonding In Metal Carbonyls Class 12 Notes (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. Discuss the nature of bonding in metal carbonyls. The bond between the carbonyl molecule and the metal is further strengthened by the. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Chemistry Coordination Compounds part 21 (Metal Carbonyl bonds) CBSE Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (a) in coordination compounds, metals show two types of valencies : (2) when co acts as a ligand, this orbital. Discuss the nature of bonding in metal carbonyls. (1) in co molecule the highest occupied molecular orbital is a. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
23) Bonding in metal carbonyls Synergic bonding in metal carbonyls Bonding In Metal Carbonyls Class 12 Notes (a) in coordination compounds, metals show two types of valencies : (2) when co acts as a ligand, this orbital. ️📚👉 watch full free course videos: In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by. Bonding In Metal Carbonyls Class 12 Notes.
From www.studocu.com
Metal Carbonyls old Metal Carbonyls The simplest view of covalent Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Describe the nature of bonding in metal carbonyls. CLASS 12 Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. ️📚👉 watch full free course videos: (2) when co acts as a ligand, this orbital. In 1893, werner proposed a theory. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Chemistry 12 Chapter 9 Bonding in Metal Carbonyls Importance Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. (a) in coordination compounds, metals show two types of valencies : The bond between the carbonyl molecule and the metal is further strengthened by the synergic. ️📚👉 watch full free course videos: Discuss the nature of bonding in metal carbonyls. (2) when co acts as a ligand, this orbital. In 1893, werner proposed a. Bonding In Metal Carbonyls Class 12 Notes.
From askfilo.com
I9 Bonding in Metal Carbonyls , Transition metal carbonyls constitute thi.. Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Discuss the nature of bonding in metal carbonyls. ️📚👉 watch full free course videos: (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. (a) in coordination compounds, metals show two. Bonding In Metal Carbonyls Class 12 Notes.
From collegedunia.com
CBSE Class 12 Chemistry Notes Chapter 9 Coordination Compounds Bonding In Metal Carbonyls Class 12 Notes The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (a) in coordination compounds, metals show two types of valencies : ️📚👉. Bonding In Metal Carbonyls Class 12 Notes.
From askfilo.com
Bonding in Metal Carbonyls The bonding in metal carbonyls is described by.. Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (2) when co acts as a ligand, this orbital. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: ️📚👉 watch full free course videos: (1). Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in metal carbonyls class 12 coordination compounds Metal Bonding In Metal Carbonyls Class 12 Notes In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: ️📚👉 watch full free course videos: Discuss the nature of bonding in metal carbonyls. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. (a) in coordination compounds, metals show two types of valencies : The bond. Bonding In Metal Carbonyls Class 12 Notes.
From classnotes.org.in
Metal Carbonyls Chemistry, Class 12, Coordination Compounds Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (a) in coordination compounds, metals show two types of valencies : The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (1) in co molecule the highest occupied molecular orbital is a. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Metal Carbonyls Bonding Structure YouTube Bonding In Metal Carbonyls Class 12 Notes Discuss the nature of bonding in metal carbonyls. (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (1) in co molecule the highest occupied molecular orbital is a. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in metal Carbonyls/TN/12th Chemistry/Unit 5/ Coordination Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: The bond between the carbonyl molecule and the metal is further strengthened by the synergic. In 1893, werner proposed a theory to explain the structure and bonding in. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Coordination Compounds Class 12 Bonding in Metal Carbonyls L12 Bonding In Metal Carbonyls Class 12 Notes (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Discuss the nature of bonding in metal carbonyls. (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies. Bonding In Metal Carbonyls Class 12 Notes.
From www.studocu.com
Metal carbonyls and bonding chemistry Studocu Bonding In Metal Carbonyls Class 12 Notes ️📚👉 watch full free course videos: (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Discuss the nature of bonding in metal carbonyls. In 1893, werner proposed a theory to. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Coordination compound part9 class 12th Bonding in metal carbonyl YouTube Bonding In Metal Carbonyls Class 12 Notes (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: The bond between the carbonyl molecule and the metal is. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in Metal CarbonylsTN Class12Very Important 5mark YouTube Bonding In Metal Carbonyls Class 12 Notes In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: ️📚👉 watch full free course videos: (2) when co acts as a ligand, this orbital. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and the metal is further strengthened. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
COORDINATION COMPOUNDS CLASS 12 JEE/NEET Class 12 Crystal Field Bonding In Metal Carbonyls Class 12 Notes (a) in coordination compounds, metals show two types of valencies : Know about the synergic effect and. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. ️📚👉 watch full free course videos: Discuss the nature of bonding in metal carbonyls. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting. Bonding In Metal Carbonyls Class 12 Notes.
From www.allaboutchemistry.net
Carbonyl CompoundsClass 12MCQs All About Chemistry Bonding In Metal Carbonyls Class 12 Notes (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. Know about the synergic effect and. ️📚👉 watch full free course videos: (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show. Bonding In Metal Carbonyls Class 12 Notes.
From www.allaboutchemistry.net
Carbonyl CompoundsClass 12MCQs All About Chemistry Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. (a) in coordination compounds, metals show two types of valencies : (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. In 1893, werner proposed a theory to explain the structure. Bonding In Metal Carbonyls Class 12 Notes.
From www.studypool.com
SOLUTION Metal carbonyls notes with details and structures Studypool Bonding In Metal Carbonyls Class 12 Notes ️📚👉 watch full free course videos: (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies : In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. Discuss the nature of bonding in metal carbonyls. The bond between the carbonyl. Bonding In Metal Carbonyls Class 12 Notes.
From byjus.com
60 How to compare the C O bond lengths in metal carbonyls? Bonding In Metal Carbonyls Class 12 Notes (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: Know about the synergic effect and. (a) in coordination compounds, metals show two types of valencies : The bond between the carbonyl molecule and the metal is. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in Metal Carbonyls Part 36 Unit 9 CBSE grade 12 chemistry Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. (a) in coordination compounds, metals show two types of valencies : ️📚👉 watch full free course videos: Discuss the nature of bonding in metal carbonyls. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (1) in co molecule the highest occupied molecular orbital is a lone pair projecting. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Class XII, Subject Chemistry, Topic BONDING IN METAL CARBONYLS Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. Discuss the nature of bonding in metal carbonyls. ️📚👉 watch full free course videos: (2) when co acts as a ligand, this orbital. The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Synergic Bonding in Metal carbonyl Coordination Compounds Chemistry Bonding In Metal Carbonyls Class 12 Notes Know about the synergic effect and. In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (a) in coordination compounds, metals show two types of valencies : The bond between the carbonyl molecule and the metal is further strengthened by the synergic. (2) when co acts as a ligand, this orbital. (1) in co molecule. Bonding In Metal Carbonyls Class 12 Notes.
From www.youtube.com
Bonding in Metal Carbonyls Coordination Compounds Class 12 Bonding In Metal Carbonyls Class 12 Notes In 1893, werner proposed a theory to explain the structure and bonding in coordination compounds: (2) when co acts as a ligand, this orbital. (a) in coordination compounds, metals show two types of valencies : (1) in co molecule the highest occupied molecular orbital is a lone pair projecting away from carbon atom. The bond between the carbonyl molecule and. Bonding In Metal Carbonyls Class 12 Notes.