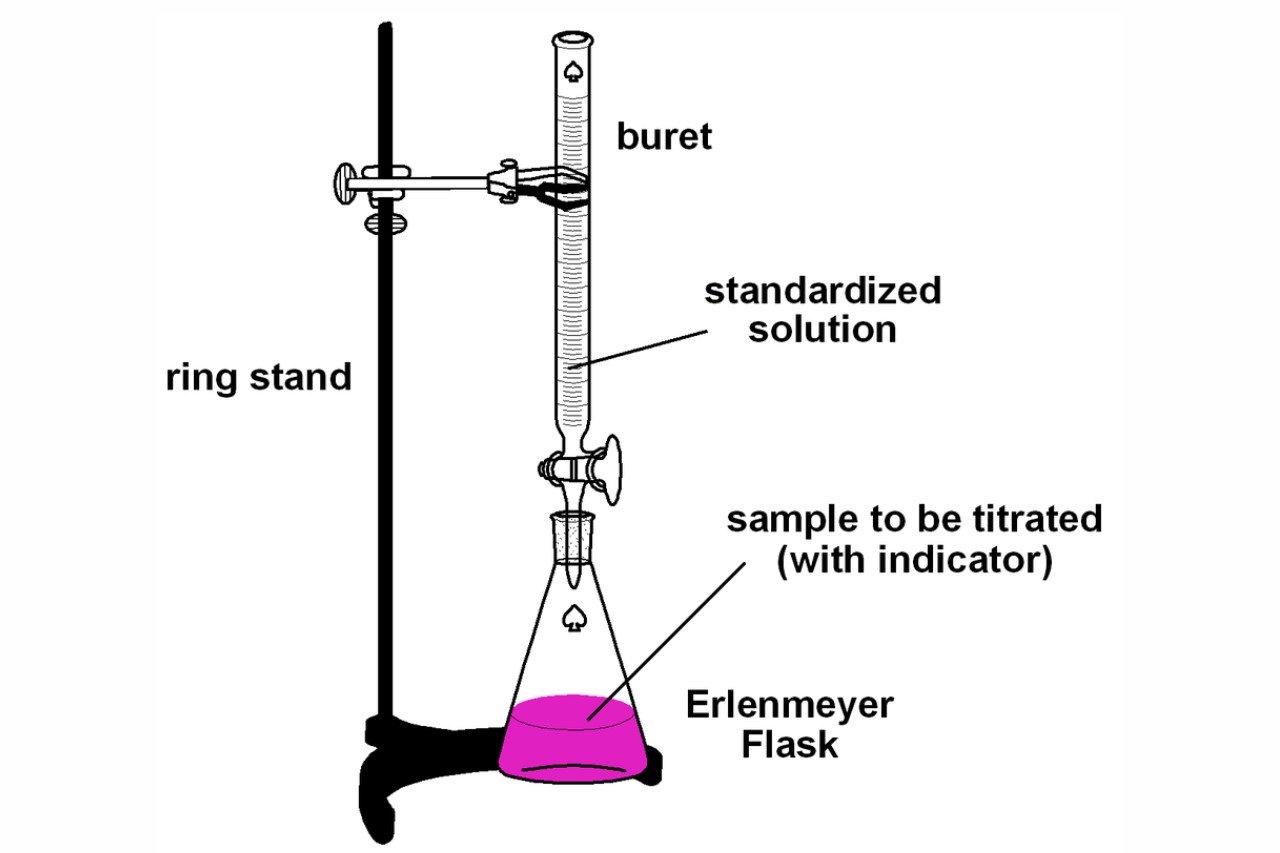
Titration Paper . By the end of this section, you will be able to: Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In this chapter, we complete our preceding study of titration curves. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. Ph can be assessed by litmus. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. The process is usually carried out by gradually adding. In particular, we set up the exact equations of the titration.
from facts.net
Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. The process is usually carried out by gradually adding. Ph can be assessed by litmus. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. In this chapter, we complete our preceding study of titration curves. In particular, we set up the exact equations of the titration. By the end of this section, you will be able to:
8 Captivating Facts About AcidBase Titration
Titration Paper By the end of this section, you will be able to: In this chapter, we complete our preceding study of titration curves. In particular, we set up the exact equations of the titration. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. The process is usually carried out by gradually adding. Ph can be assessed by litmus. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. By the end of this section, you will be able to:
From www.etsy.com
Nursing Drip Titration Flowsheet Etsy Titration Paper The process is usually carried out by gradually adding. Ph can be assessed by litmus. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph,. Titration Paper.
From www.vrogue.co
Chemistry Notes Igcse Gcse Olevel Titration Igcse Gcs vrogue.co Titration Paper By the end of this section, you will be able to: In this chapter, we complete our preceding study of titration curves. Ph can be assessed by litmus. The process is usually carried out by gradually adding. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Titration is a. Titration Paper.
From www.vrogue.co
Solved Draw A Titration Curve For Ala Use Figure 3 10 vrogue.co Titration Paper The process is usually carried out by gradually adding. In this chapter, we complete our preceding study of titration curves. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up the exact equations of the titration. Titration of an acid with a. Titration Paper.
From mungfali.com
Acid Base Titration Calculation Titration Paper In particular, we set up the exact equations of the titration. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. The process is usually carried. Titration Paper.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog Titration Paper Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Ph can be assessed by litmus. By the. Titration Paper.
From www.shutterstock.com
1348 wynik(i/ów) dla „Titration experiment” w kategorii obrazy Titration Paper Ph can be assessed by litmus. In this chapter, we complete our preceding study of titration curves. The process is usually carried out by gradually adding. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. Titration of an acid with a base requires that the ph, or relative. Titration Paper.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up the exact equations of the titration. By the end of this section, you will be able to: Ph can be assessed by litmus. The process is usually carried out by gradually adding.. Titration Paper.
From justinmsiebert.com
Graphing WASB Titration Curves on Giant Graph Paper Siebert Science Titration Paper The process is usually carried out by gradually adding. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point. Titration Paper.
From www.alamy.com
Scientist performing titration in a chemistry laboratory with Redox Titration Paper In particular, we set up the exact equations of the titration. Ph can be assessed by litmus. By the end of this section, you will be able to: Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph meter/instrument and a glass. Titration Paper.
From www.coursehero.com
[Solved] Draw the appropriate titration curve for the tripeptide Met Titration Paper Ph can be assessed by litmus. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. By the end of this section, you will be able to: Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored.. Titration Paper.
From boardtopper.blogspot.com
Titration 1 Class 12 CBSE Chemistry Practical All Study Guide at Titration Paper In particular, we set up the exact equations of the titration. In this chapter, we complete our preceding study of titration curves. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. By the end of this section, you will be able to: Learn how to measure ph using. Titration Paper.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Paper In this chapter, we complete our preceding study of titration curves. The process is usually carried out by gradually adding. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. By the end of this section, you will be able to: In particular, we set up the. Titration Paper.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Paper Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph. Titration Paper.
From www.studocu.com
Titration 4 Plus Two Chemistry Record CBSE Organic Chemistry MG Titration Paper In particular, we set up the exact equations of the titration. The process is usually carried out by gradually adding. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. By the end of this section, you will be able to: In this chapter, we complete our preceding study. Titration Paper.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Paper In particular, we set up the exact equations of the titration. By the end of this section, you will be able to: Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In this chapter, we complete our preceding study of titration curves. Titration is a common. Titration Paper.
From www.scribd.com
Chemistry Paper 3 PDF PDF Titration Chemistry Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. By the end of this section, you will be able to: In particular, we set up. Titration Paper.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Ph can be assessed by litmus. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored.. Titration Paper.
From www.etsy.com
Buy Nursing Drip Titration Flowsheet Online in India Etsy Titration Paper Ph can be assessed by litmus. By the end of this section, you will be able to: The process is usually carried out by gradually adding. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Titration of an acid with a base requires that the ph,. Titration Paper.
From www.studocu.com
Aspirin tablets titration Titration of Aspirin Tablets In this lab Titration Paper By the end of this section, you will be able to: In particular, we set up the exact equations of the titration. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Titration of an acid with a base requires that the ph, or relative concentrations of. Titration Paper.
From phdessay.com
Titration Research Paper Titration Paper In this chapter, we complete our preceding study of titration curves. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Ph can be assessed by litmus. In particular, we set up the exact equations of the titration. Titration is a common laboratory method of quantitative chemical. Titration Paper.
From www.studocu.com
Titration Report BTEC Applied Science Unit2 A Titration Report Titration Paper By the end of this section, you will be able to: Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. The process is usually carried out by gradually adding. Ph can be assessed by litmus. Titration of an acid with a base requires that the ph,. Titration Paper.
From www.shutterstock.com
Titration Vector Illustration Labeled Educational Chemistry Stock Titration Paper In this chapter, we complete our preceding study of titration curves. Ph can be assessed by litmus. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. In particular, we set up the exact equations of the titration. Learn how to measure ph using a ph meter/instrument and a glass. Titration Paper.
From www.chegg.com
Solved QUESTION 1 A grade 12 learner performed a titration Titration Paper Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. In this chapter, we complete our preceding study of titration curves. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up. Titration Paper.
From www.etsy.com
Titration Flowsheet, Medication Titration Sheet, ICU Nurse, RN, BSN Etsy Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Ph can be assessed by litmus. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Titration is a common laboratory method of quantitative chemical analysis that. Titration Paper.
From www.vrogue.co
Acid Base Titration In Bangal vrogue.co Titration Paper Ph can be assessed by litmus. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. By the end of this section, you will be able to:. Titration Paper.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Paper In this chapter, we complete our preceding study of titration curves. The process is usually carried out by gradually adding. Ph can be assessed by litmus. In particular, we set up the exact equations of the titration. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration.. Titration Paper.
From ar.inspiredpencil.com
Titration Diagram Titration Paper In particular, we set up the exact equations of the titration. In this chapter, we complete our preceding study of titration curves. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. The process is usually carried out by gradually adding. By the end of this section, you will. Titration Paper.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Paper Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. In particular, we set up the exact equations of the titration. By the end of this section, you will be able to: In this chapter, we complete our preceding study of titration curves. Ph can be assessed by litmus. Learn. Titration Paper.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. In this chapter, we complete our. Titration Paper.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Paper By the end of this section, you will be able to: In this chapter, we complete our preceding study of titration curves. The process is usually carried out by gradually adding. In particular, we set up the exact equations of the titration. Ph can be assessed by litmus. Learn how to measure ph using a ph meter/instrument and a glass. Titration Paper.
From www.youtube.com
Chemistry How to do Titrations for Paper 3 // 9701/33/O/N/22 YouTube Titration Paper Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In this chapter, we complete our preceding study of titration curves. Ph can be assessed by litmus.. Titration Paper.
From users.highland.edu
AcidBase Titrations Titration Paper By the end of this section, you will be able to: Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. Ph can be assessed by litmus.. Titration Paper.
From tomschoderbekchem.blogspot.com
Tom Schoderbek Chemistry Titration Paper Lab Titration Paper Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Ph can be assessed by litmus. The process is usually carried out by gradually adding. By the end of this section, you will be able to: In this chapter, we complete our preceding study of titration curves. Titration is a. Titration Paper.
From www.studypool.com
SOLUTION Standardization of Acids and Bases Titration Paper Studypool Titration Paper Learn how to measure ph using a ph meter/instrument and a glass electrode, and how to determine the equivalence point of a titration. In particular, we set up the exact equations of the titration. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be monitored. Titration is a common laboratory method. Titration Paper.
From www.studocu.com
Titration questions from past papers chem102 Monash Studocu Titration Paper The process is usually carried out by gradually adding. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. In this chapter, we complete our preceding study of titration curves. Titration of an acid with a base requires that the ph, or relative concentrations of the two reactants, be. Titration Paper.