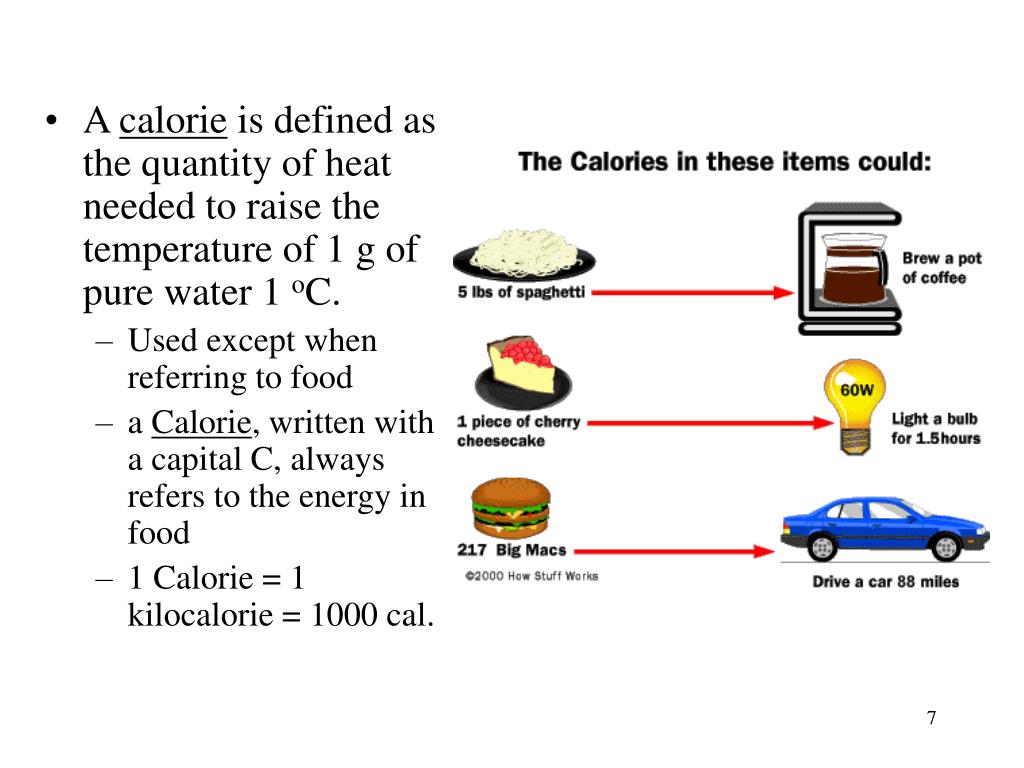
Calories In Heat Energy . A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. It is commonly used as the unit for heat. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. To measure the number of calories in a particular food. For precise work, however, it becomes.
from www.slideserve.com
For precise work, however, it becomes. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. To measure the number of calories in a particular food. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. It is commonly used as the unit for heat. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules.
PPT Chapter 11 PowerPoint Presentation, free download ID1084959
Calories In Heat Energy One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. For precise work, however, it becomes. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. It is commonly used as the unit for heat. To measure the number of calories in a particular food. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian.
From slideplayer.com
Thermochemistry The study of the changes in heat energy that chemical reactions and Calories In Heat Energy One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. For precise work, however, it becomes. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Roughly, the calorie is the heat. Calories In Heat Energy.
From studylib.net
Heat Equation Calories In Heat Energy To measure the number of calories in a particular food. For precise work, however, it becomes. It is commonly used as the unit for heat. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. Of water by 1.8 degrees fahrenheit), said grace derocha,. Calories In Heat Energy.
From www.showme.com
Heat capacity turning to calories Science, Chemistry ShowMe Calories In Heat Energy Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. To measure the number of calories in a particular food. For precise work, however, it becomes. A calorie is. Calories In Heat Energy.
From fitnessprogramer.com
Understanding Energy And Calories / Workout Builder Calories In Heat Energy A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. To measure the number of calories in a particular food. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. Calories In Heat Energy.
From www.youtube.com
What is a Calorie? Explained in terms of Energy, Food & Calorie Burn YouTube Calories In Heat Energy Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. It is commonly used as. Calories In Heat Energy.
From studylib.net
Heat Energy Chemistry Calories In Heat Energy To measure the number of calories in a particular food. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. A unit of heat energy used. Calories In Heat Energy.
From slideplayer.com
Heat Engines Lecture 6 HNRS 228 Energy and the Environment. ppt download Calories In Heat Energy A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. It is commonly used as the unit for heat. To measure the number of calories in a particular food. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. Another common unit of energy. Calories In Heat Energy.
From slideplayer.com
Measuring Food Energy by Calorimetry ppt download Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Another common unit of energy often used for heat is the calorie (cal), defined as the. Calories In Heat Energy.
From www.verywellfit.com
Figuring Out How Many Calories You Burn Every Day Calories In Heat Energy For precise work, however, it becomes. It is commonly used as the unit for heat. To measure the number of calories in a particular food. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. A unit of. Calories In Heat Energy.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID6836296 Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. For precise work, however, it becomes. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius. Calories In Heat Energy.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calories In Heat Energy For precise work, however, it becomes. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed. Calories In Heat Energy.
From www.tessshebaylo.com
Use The Heat Equation To Calculate Energy In Joules And Calories For Each Of Following Calories In Heat Energy Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. It is commonly used as the unit for heat. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to. Calories In Heat Energy.
From slideplayer.com
Nutrition Notes. ppt download Calories In Heat Energy A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Another common unit of energy often used for. Calories In Heat Energy.
From www.slideshare.net
Heat and Calorie Calories In Heat Energy A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. For precise work, however, it becomes. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. To measure the number of calories in a particular. Calories In Heat Energy.
From byjus.com
in a process, 500 calories of heat is given to a system and at the same time 100 joules of work Calories In Heat Energy Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. A calorie. Calories In Heat Energy.
From becuo.com
Heat Energy Images & Pictures Becuo Calories In Heat Energy Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. For precise work, however, it becomes. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature. Calories In Heat Energy.
From studylib.net
1. A calorie is a unit of energy OG Calories In Heat Energy Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. To measure the number of calories in a particular food. A unit of heat energy used in thermochemistry is. Calories In Heat Energy.
From www.chegg.com
Solved Match the gain or loss of heat energy with the Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. To measure the number of calories in a particular food. A calorie is the. Calories In Heat Energy.
From www.slideserve.com
PPT Energy Notes (cont.) PowerPoint Presentation, free download ID7086427 Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. For precise work, however, it becomes. To measure the number of calories in a. Calories In Heat Energy.
From medicinebtg.com
define Calories Definition calorie a unit of heat used to indicate the amount energy that foods Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. For precise work, however, it becomes. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. A unit of heat energy used in thermochemistry. Calories In Heat Energy.
From yccac.org
Heat, Energy and Fuel York County Community Action Corp. Calories In Heat Energy A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. For precise work, however, it becomes. It is commonly used as the unit for heat. Of water by 1.8 degrees fahrenheit), said grace derocha,. Calories In Heat Energy.
From www.slideshare.net
Heat Energy Calories In Heat Energy Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. Roughly, the calorie is the. Calories In Heat Energy.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation, free download ID4051034 Calories In Heat Energy Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. Another common unit of energy often used for. Calories In Heat Energy.
From www.slideserve.com
PPT Heat & Thermodynamics PowerPoint Presentation, free download ID6534487 Calories In Heat Energy For precise work, however, it becomes. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. It is commonly used as the unit for heat. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. A unit of heat energy. Calories In Heat Energy.
From gamesmartz.com
Calorie Definition & Image GameSmartz Calories In Heat Energy To measure the number of calories in a particular food. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to. Calories In Heat Energy.
From www.youtube.com
Unit of heat energy SI unit of heat CGS unit of heat Joule calorie BTU What is heat Calories In Heat Energy Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. One kcal is. Calories In Heat Energy.
From slideplayer.com
Thermal Energy and Heat ppt download Calories In Heat Energy To measure the number of calories in a particular food. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius.. Calories In Heat Energy.
From slideplayer.com
Unit 5 Thermochemistry ppt download Calories In Heat Energy To measure the number of calories in a particular food. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. A calorie is the amount of energy in the form of heat that is. Calories In Heat Energy.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation, free download ID1278045 Calories In Heat Energy One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. For precise work, however, it becomes. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one. Calories In Heat Energy.
From www.slideshare.net
Heat and Calorie Calories In Heat Energy A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. For precise work, however, it becomes. One kcal is the amount of energy required to heat. Calories In Heat Energy.
From www.youtube.com
Joules, Food Calories, & Kilojoules Unit Conversion With Heat Energy Physics Problems YouTube Calories In Heat Energy A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. For precise work, however, it becomes. It is commonly used as the unit for heat. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. A calorie is. Calories In Heat Energy.
From slideplayer.com
Chemical Pathways Chapter ppt download Calories In Heat Energy For precise work, however, it becomes. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of. A unit of heat. Calories In Heat Energy.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID5760246 Calories In Heat Energy Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically,. To measure the number of calories in a particular food. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Roughly, the calorie is the. Calories In Heat Energy.
From www.slideserve.com
PPT Temperature, Heat, and Expansion PowerPoint Presentation, free download ID2137513 Calories In Heat Energy For precise work, however, it becomes. One kcal is the amount of energy required to heat 1 kilogram of water by 1 degree celsius (2.2 lbs. Of water by 1.8 degrees fahrenheit), said grace derocha, a registered dietitian. A unit of heat energy used in thermochemistry is the thermochemical calorie, equal to 4.184 joules. Another common unit of energy often. Calories In Heat Energy.
From www.slideserve.com
PPT Chapter 11 Energy in Thermal Process PowerPoint Presentation, free download ID1133445 Calories In Heat Energy Roughly, the calorie is the heat required to raise the temperature of a gram of water through 1 c o. A calorie is the amount of energy in the form of heat that is required to heat one gram of water one degree celsius. To measure the number of calories in a particular food. One kcal is the amount of. Calories In Heat Energy.