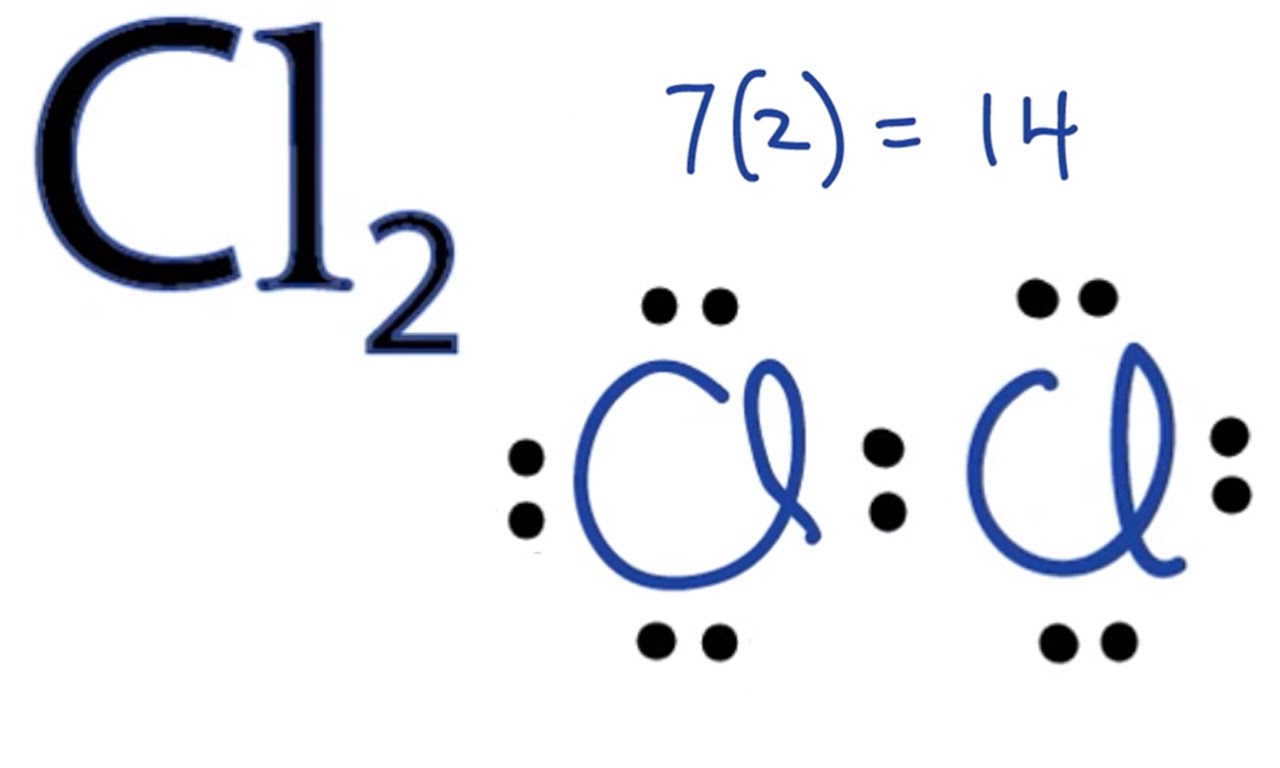Chlorine Ion Electrons . Electron configuration of chlorine is [ne]. But a chlorine ion (cl−) has. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). A chlorine atom starts with. Following aufbau's principle, the electron. The electron configuration of a chlorine atom (cl) is as follows: Let’s take a look at how it is formed. Sodium chloride, or table salt, is an ionic compound. Neutral chlorine atom on left has 17 protons and 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)).
from periodictable.me
The electron configuration of a chlorine atom (cl) is as follows: A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Let’s take a look at how it is formed. Following aufbau's principle, the electron. But a chlorine ion (cl−) has. Sodium chloride, or table salt, is an ionic compound. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Electron configuration of chlorine is [ne].
Chlorine Electron Configuration (Cl) with Orbital Diagram
Chlorine Ion Electrons Sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). But a chlorine ion (cl−) has. A chlorine atom starts with. Sodium chloride, or table salt, is an ionic compound. The electron configuration of a chlorine atom (cl) is as follows: Electron configuration of chlorine is [ne]. Following aufbau's principle, the electron. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Neutral chlorine atom on left has 17 protons and 17 electrons. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)).
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Electrons Electron configuration of chlorine is [ne]. Neutral chlorine atom on left has 17 protons and 17 electrons. Let’s take a look at how it is formed. The electron configuration of a chlorine atom (cl) is as follows: Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−) has. A chlorine atom always gains one electron when. Chlorine Ion Electrons.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Shutterstock Chlorine Ion Electrons Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−) has. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Electron configuration of. Chlorine Ion Electrons.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Larry Alford blog Chlorine Ion Electrons A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne]. Sodium chloride, or table salt, is an ionic compound. During. Chlorine Ion Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Ion Electrons In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne]. The electron configuration of a chlorine atom (cl) is as follows: Following aufbau's principle, the electron. During the formation of sodium chloride, the electron given off by sodium is. Chlorine Ion Electrons.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Ion Electrons The electron configuration of a chlorine atom (cl) is as follows: Neutral chlorine atom on left has 17 protons and 17 electrons. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17. Chlorine Ion Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Ion Electrons A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−) has. Electron configuration of chlorine is [ne]. Following aufbau's principle, the electron. In order to write the chlorine electron configuration we first need to know the number. Chlorine Ion Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Electrons A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Following aufbau's principle, the electron. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom starts with. But a chlorine ion (cl−) has. The electron configuration of a chlorine atom (cl) is as follows: Electron configuration. Chlorine Ion Electrons.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ion Electrons The electron configuration of a chlorine atom (cl) is as follows: Following aufbau's principle, the electron. Neutral chlorine atom on left has 17 protons and 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Electron configuration. Chlorine Ion Electrons.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Ion Electrons A chlorine atom starts with. But a chlorine ion (cl−) has. Following aufbau's principle, the electron. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium chloride, or table salt, is an ionic compound. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17. Chlorine Ion Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Ion Electrons Electron configuration of chlorine is [ne]. Let’s take a look at how it is formed. Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Sodium chloride, or table salt, is an ionic compound.. Chlorine Ion Electrons.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral at... Physical Chemistry Chlorine Ion Electrons A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The electron configuration of a chlorine atom (cl) is as follows: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Sodium chloride, or table salt, is an ionic compound. Electron. Chlorine Ion Electrons.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Shutterstock Chlorine Ion Electrons Following aufbau's principle, the electron. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium chloride, or table salt, is an ionic compound. A chlorine atom starts with. But a chlorine ion (cl−) has. Let’s take a look at how it is formed. The electron configuration of a chlorine atom (cl) is as follows: Electron configuration of chlorine. Chlorine Ion Electrons.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation ID3462514 Chlorine Ion Electrons In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). But a chlorine ion (cl−) has. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. The electron configuration of a chlorine atom (cl) is as follows: Neutral. Chlorine Ion Electrons.
From pixels.com
Chlorine Electron Configuration Photograph by Photo Libary Pixels Chlorine Ion Electrons A chlorine atom starts with. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electron configuration of a chlorine atom (cl) is as follows: Electron configuration. Chlorine Ion Electrons.
From www.science-revision.co.uk
Atoms to ions Chlorine Ion Electrons In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Electron configuration of chlorine is [ne]. Let’s take a look at how it is formed. Neutral. Chlorine Ion Electrons.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Ion Electrons During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Let’s take a look at how it is formed. Electron configuration of chlorine is [ne]. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−). Chlorine Ion Electrons.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Electrons A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. But a chlorine ion (cl−) has. In order to write the chlorine electron configuration we first need to know the. Chlorine Ion Electrons.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Ion Electrons Let’s take a look at how it is formed. But a chlorine ion (cl−) has. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom starts with. Electron configuration of chlorine is [ne]. Sodium chloride, or table salt, is an ionic compound. Following aufbau's principle, the electron. In order to. Chlorine Ion Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Ion Electrons In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Electron configuration of. Chlorine Ion Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Ion Electrons A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Sodium chloride, or table salt, is an ionic compound. The electron configuration of a chlorine atom (cl) is as follows: Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number. Chlorine Ion Electrons.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Chlorine Ion Electrons The electron configuration of a chlorine atom (cl) is as follows: Electron configuration of chlorine is [ne]. Let’s take a look at how it is formed. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). But a chlorine ion (cl−) has. Following aufbau's principle,. Chlorine Ion Electrons.
From sciencenotes.org
Chlorine Facts Chlorine Ion Electrons But a chlorine ion (cl−) has. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Sodium chloride, or table salt, is an ionic compound. A chlorine atom starts with. Electron. Chlorine Ion Electrons.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Ion Electrons Let’s take a look at how it is formed. Electron configuration of chlorine is [ne]. Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). During the formation of sodium chloride, the electron given. Chlorine Ion Electrons.
From gardenandplate.com
Molecules Chlorine Ion Electrons The electron configuration of a chlorine atom (cl) is as follows: A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−) has. During the formation of sodium chloride, the electron given off by sodium is taken by. Chlorine Ion Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Ion Electrons But a chlorine ion (cl−) has. Sodium chloride, or table salt, is an ionic compound. Electron configuration of chlorine is [ne]. Following aufbau's principle, the electron. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The electron configuration of a chlorine atom (cl) is as follows: Let’s take a look at how it is formed.. Chlorine Ion Electrons.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Ion Electrons During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Neutral chlorine atom on left has 17 protons and 17 electrons. Following aufbau's principle, the electron. A chlorine atom starts with. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Chlorine Ion Electrons.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − ) when it gains an Chlorine Ion Electrons Electron configuration of chlorine is [ne]. Following aufbau's principle, the electron. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17. Chlorine Ion Electrons.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Ion Electrons But a chlorine ion (cl−) has. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Following aufbau's principle, the electron. A chlorine atom starts with. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Electron configuration of chlorine is. Chlorine Ion Electrons.
From ar.inspiredpencil.com
Electron Configuration For Chlorine Chlorine Ion Electrons The electron configuration of a chlorine atom (cl) is as follows: Electron configuration of chlorine is [ne]. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Neutral chlorine atom on left has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number. Chlorine Ion Electrons.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Ion Electrons In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Neutral chlorine atom on left has 17 protons and 17 electrons. A chlorine atom starts with. But a chlorine ion (cl−) has. Sodium chloride, or table salt, is an ionic compound. The electron configuration of. Chlorine Ion Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Ion Electrons During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. But a chlorine ion (cl−) has. Following aufbau's principle, the electron. Neutral chlorine atom on left has 17 protons and 17 electrons. Electron configuration of chlorine is [ne]. A chlorine atom starts with. The electron configuration of a chlorine atom (cl) is as. Chlorine Ion Electrons.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Ion Electrons Let’s take a look at how it is formed. Sodium chloride, or table salt, is an ionic compound. Neutral chlorine atom on left has 17 protons and 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)).. Chlorine Ion Electrons.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Ion Electrons Sodium chloride, or table salt, is an ionic compound. But a chlorine ion (cl−) has. Neutral chlorine atom on left has 17 protons and 17 electrons. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Electron configuration of chlorine is [ne]. The electron. Chlorine Ion Electrons.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Ion Electrons Let’s take a look at how it is formed. Neutral chlorine atom on left has 17 protons and 17 electrons. Following aufbau's principle, the electron. Sodium chloride, or table salt, is an ionic compound. A chlorine atom starts with. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. The electron configuration of. Chlorine Ion Electrons.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Ion Electrons During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. A chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Neutral chlorine atom on left has 17 protons and 17 electrons. But a chlorine ion (cl−) has. Electron configuration of chlorine is [ne]. The electron configuration of a chlorine. Chlorine Ion Electrons.