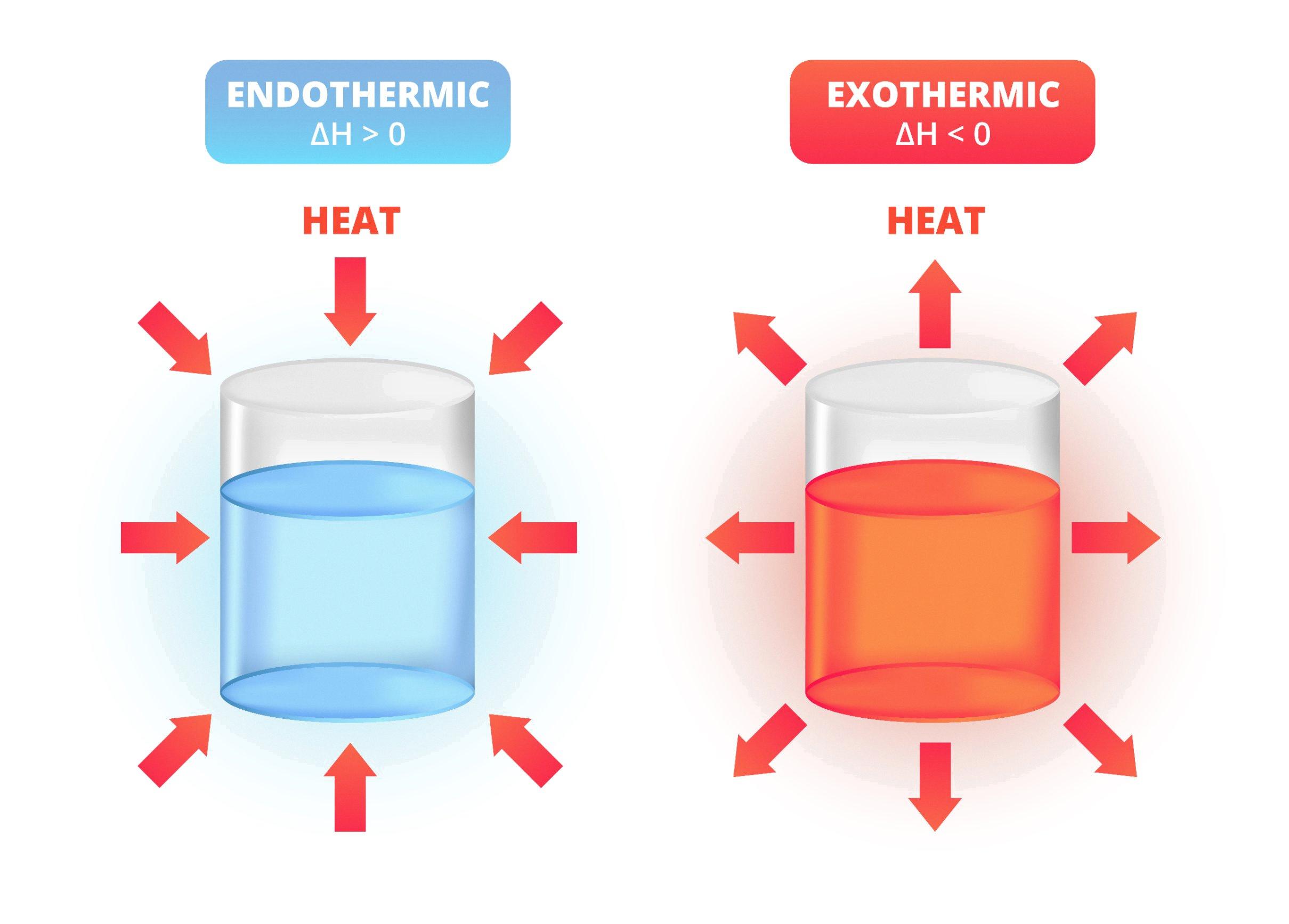
Endothermic Process Example Chemical Reaction . The heat is absorbed from the surroundings allowing the reactants to transform into products. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The absorbed energy provides the activation energy for the reaction to. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.
from h-o-m-e.org
The absorbed energy provides the activation energy for the reaction to. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The heat is absorbed from the surroundings allowing the reactants to transform into products. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings.
Endothermic Reactions The Science Behind Temperature Change
Endothermic Process Example Chemical Reaction An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. The absorbed energy provides the activation energy for the reaction to. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the surroundings allowing the reactants to transform into products. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is any chemical reaction that absorbs heat from its environment. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The opposite of an endothermic reaction is an exothermic reaction.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704 Endothermic Process Example Chemical Reaction The opposite of an endothermic reaction is an exothermic reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A chemical reaction or. Endothermic Process Example Chemical Reaction.
From printablelibzeloso.z21.web.core.windows.net
Endothermic Vs Exothermic Reactions Examples Endothermic Process Example Chemical Reaction The opposite of an endothermic reaction is an exothermic reaction. The absorbed energy provides the activation energy for the reaction to. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the surroundings allowing the reactants to transform into products. An endothermic reaction is a type of chemical reaction during which. Endothermic Process Example Chemical Reaction.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. The heat is absorbed from the surroundings allowing the reactants to transform into products. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment. Endothermic Process Example Chemical Reaction.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Process Example Chemical Reaction One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. The heat is absorbed from the surroundings allowing the reactants to transform into products. A chemical reaction is. Endothermic Process Example Chemical Reaction.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Process Example Chemical Reaction When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. The heat is absorbed from the surroundings allowing the reactants to transform into products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the. Endothermic Process Example Chemical Reaction.
From www.slideserve.com
PPT Chemistry Chemical Reactions Exothermic and Endothermic PowerPoint Presentation ID3894482 Endothermic Process Example Chemical Reaction The absorbed energy provides the activation energy for the reaction to. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The heat is absorbed from the surroundings allowing the reactants to transform into products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. When. Endothermic Process Example Chemical Reaction.
From mavink.com
Endothermic And Exothermic Reactions Endothermic Process Example Chemical Reaction The opposite of an endothermic reaction is an exothermic reaction. The absorbed energy provides the activation energy for the reaction to. An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the surroundings allowing the reactants to transform. Endothermic Process Example Chemical Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Process Example Chemical Reaction For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. A chemical reaction or physical change. Endothermic Process Example Chemical Reaction.
From www.dreamstime.com
Endothermic Reaction and Exothermic Process Stock Vector Illustration of absorb, physics Endothermic Process Example Chemical Reaction For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The opposite of an endothermic reaction is an exothermic reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a type of chemical reaction during which a system absorbs energy. Endothermic Process Example Chemical Reaction.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. The heat is absorbed from the surroundings allowing the reactants to transform into products. The absorbed energy provides the activation energy for the reaction to. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a type of. Endothermic Process Example Chemical Reaction.
From slimpixs.blogspot.com
Endothermic And Exothermic Reactions Endothermic Reaction Examples Many processes that Endothermic Process Example Chemical Reaction The heat is absorbed from the surroundings allowing the reactants to transform into products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is any chemical reaction that absorbs heat from its. Endothermic Process Example Chemical Reaction.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Process Example Chemical Reaction A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. The heat is absorbed from the surroundings allowing the reactants to transform into products. An endothermic reaction is any chemical reaction that absorbs heat. Endothermic Process Example Chemical Reaction.
From klasgnqsj.blob.core.windows.net
Endothermic Reaction Graph Example at Britt Fields blog Endothermic Process Example Chemical Reaction The heat is absorbed from the surroundings allowing the reactants to transform into products. An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Endothermic Process Example Chemical Reaction.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a type of chemical reaction during. Endothermic Process Example Chemical Reaction.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Process Example Chemical Reaction A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The absorbed energy provides the activation energy for the reaction to. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. The opposite of an endothermic reaction is an exothermic reaction. An endothermic. Endothermic Process Example Chemical Reaction.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. The opposite of. Endothermic Process Example Chemical Reaction.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Process Example Chemical Reaction A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. The absorbed energy provides the activation energy for. Endothermic Process Example Chemical Reaction.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Process Example Chemical Reaction A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The opposite of an endothermic reaction is an exothermic reaction. The heat is absorbed from. Endothermic Process Example Chemical Reaction.
From klasgnqsj.blob.core.windows.net
Endothermic Reaction Graph Example at Britt Fields blog Endothermic Process Example Chemical Reaction The opposite of an endothermic reaction is an exothermic reaction. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is any chemical reaction that absorbs heat from its environment.. Endothermic Process Example Chemical Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Process Example Chemical Reaction A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. When ammonium chloride (nh 4. Endothermic Process Example Chemical Reaction.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Process Example Chemical Reaction The absorbed energy provides the activation energy for the reaction to. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The heat is absorbed from the surroundings allowing the reactants to transform into products. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. A chemical reaction is. Endothermic Process Example Chemical Reaction.
From exywpuoix.blob.core.windows.net
Ice Pack Endothermic Or Exothermic Reaction at Vivian Dougherty blog Endothermic Process Example Chemical Reaction A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. One can also say that in the case of an endothermic process, the. Endothermic Process Example Chemical Reaction.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and equation YouTube Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. The heat is absorbed from the surroundings allowing the reactants to transform. Endothermic Process Example Chemical Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Process Example Chemical Reaction One can also say that in the case of an endothermic process, the change in enthalpy is always positive. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants. Endothermic Process Example Chemical Reaction.
From www.scigallery.com
GCSE Chemistry Mindmap Exothermic and Endothermic Reactions (example) Endothermic Process Example Chemical Reaction The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to transform into products. The opposite of an endothermic reaction is an exothermic reaction. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. When ammonium chloride (nh 4. Endothermic Process Example Chemical Reaction.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction. Endothermic Process Example Chemical Reaction.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Process Example Chemical Reaction The heat is absorbed from the surroundings allowing the reactants to transform into products. The opposite of an endothermic reaction is an exothermic reaction. The absorbed energy provides the activation energy for the reaction to. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is any chemical. Endothermic Process Example Chemical Reaction.
From signalticket9.pythonanywhere.com
Impressive Endothermic Reaction Example Equation Chemical And Class 10 Notes Ncert Endothermic Process Example Chemical Reaction The absorbed energy provides the activation energy for the reaction to. An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The opposite of an. Endothermic Process Example Chemical Reaction.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs Endothermic Process Example Chemical Reaction A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is any chemical reaction that absorbs heat from its environment. One can also say that in the case of an endothermic process, the change in enthalpy is always positive. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes. Endothermic Process Example Chemical Reaction.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition, Properties, Examples Endothermic Process Example Chemical Reaction When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings.. Endothermic Process Example Chemical Reaction.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Process Example Chemical Reaction One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The opposite of an endothermic reaction is an exothermic reaction. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings.. Endothermic Process Example Chemical Reaction.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction) class 10th science YouTube Endothermic Process Example Chemical Reaction One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction. Endothermic Process Example Chemical Reaction.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Process Example Chemical Reaction An endothermic reaction is any chemical reaction that absorbs heat from its environment. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their. Endothermic Process Example Chemical Reaction.
From study.com
Endothermic Reaction Definition & Example Video & Lesson Transcript Endothermic Process Example Chemical Reaction One can also say that in the case of an endothermic process, the change in enthalpy is always positive. An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is any chemical reaction that. Endothermic Process Example Chemical Reaction.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Process Example Chemical Reaction The absorbed energy provides the activation energy for the reaction to. The heat is absorbed from the surroundings allowing the reactants to transform into products. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. One can also. Endothermic Process Example Chemical Reaction.