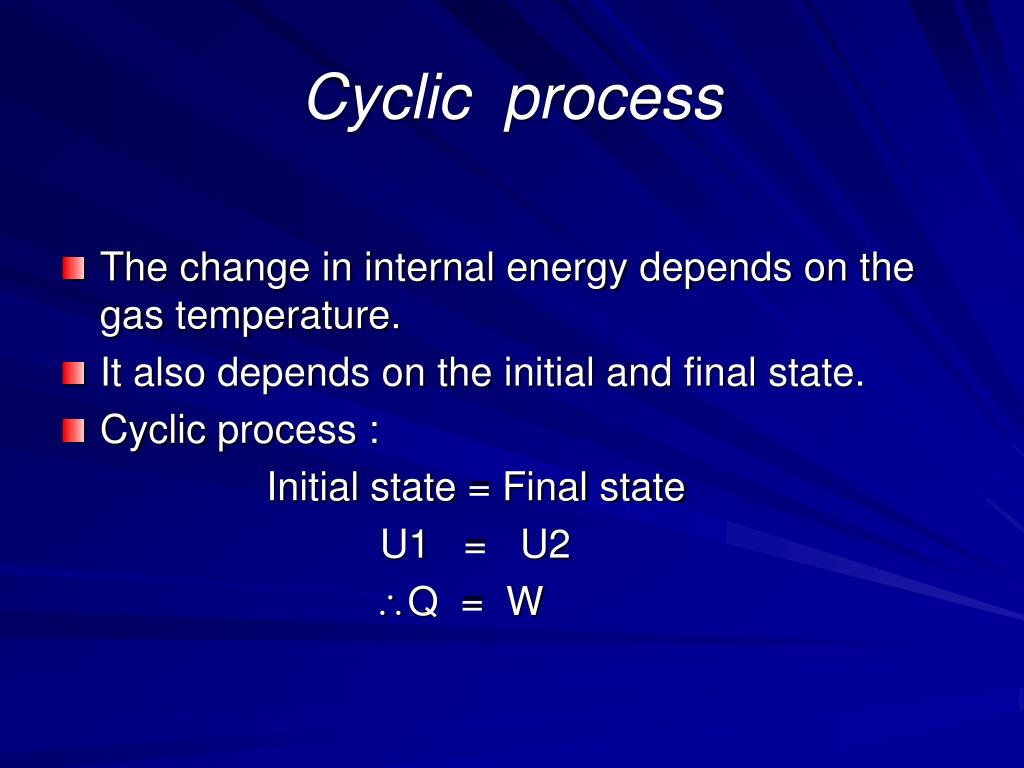
Change In Internal Energy For Cyclic Process . Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate the net work and heat in a cyclic process involving four steps: Isothermal expansion, isochoric removal of heat,. Hence, the internal energy change in any cyclic process is zero. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Understand the concepts of system, surroundings, state function,. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Applying the first law of.
from www.slideserve.com
Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Hence, the internal energy change in any cyclic process is zero. Understand the concepts of system, surroundings, state function,. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate the net work and heat in a cyclic process involving four steps: Isothermal expansion, isochoric removal of heat,. Applying the first law of. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical.
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free download ID4559065
Change In Internal Energy For Cyclic Process Isothermal expansion, isochoric removal of heat,. Understand the concepts of system, surroundings, state function,. Hence, the internal energy change in any cyclic process is zero. Learn how to calculate the net work and heat in a cyclic process involving four steps: Applying the first law of. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. When a system undergoes a cyclic process, its initial and final internal energies are equal. Isothermal expansion, isochoric removal of heat,. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical.
From slideplayer.com
Thermodynamic Processes ppt download Change In Internal Energy For Cyclic Process Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Isothermal expansion, isochoric removal of heat,. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Understand the concepts of system, surroundings, state function,. Additionally, the change in internal energy over a complete cycle. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID4342222 Change In Internal Energy For Cyclic Process Applying the first law of. Understand the concepts of system, surroundings, state function,. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Isothermal expansion, isochoric removal of heat,. Learn how to calculate the net work and heat in a cyclic process involving four steps: Calculate physical quantities, such as the heat transferred,. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1549724 Change In Internal Energy For Cyclic Process When a system undergoes a cyclic process, its initial and final internal energies are equal. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Understand the concepts of system, surroundings, state function,. Hence,. Change In Internal Energy For Cyclic Process.
From www.toppr.com
A gas is taken through the cyclic process described in Figure (a) Find the net energy Change In Internal Energy For Cyclic Process Learn how to calculate the net work and heat in a cyclic process involving four steps: Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Hence, the. Change In Internal Energy For Cyclic Process.
From www.doubtnut.com
One mole of an ideal gas undergoes a cyclic process ABCDA as shown in Change In Internal Energy For Cyclic Process Hence, the internal energy change in any cyclic process is zero. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Isothermal expansion, isochoric removal of heat,. Understand the concepts of system, surroundings, state. Change In Internal Energy For Cyclic Process.
From gazemoms.blogspot.com
The Internal Energy Of A System Is Always Increased By gazemoms Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Isothermal expansion, isochoric removal of heat,. Applying the first law of. When a system undergoes a cyclic process, its initial and final internal energies are equal. Hence, the internal energy change in any cyclic process is zero. Learn how to calculate the net work and heat in a cyclic process involving four. Change In Internal Energy For Cyclic Process.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Change In Internal Energy For Cyclic Process Isothermal expansion, isochoric removal of heat,. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Applying the first law of. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Calculate physical quantities, such as the heat transferred, work. Change In Internal Energy For Cyclic Process.
From slideplayer.com
Chapter 103 The Second Law of Thermodynamics ppt download Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Learn how to calculate the net work and heat in a cyclic process involving four steps: Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Applying the first law. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free download ID4559065 Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Applying the first law of. Learn how to calculate changes in internal energy and the flow of energy. Change In Internal Energy For Cyclic Process.
From www.youtube.com
A sample of an ideal gas is taken through the cyclic process abca as shown in the figure. The Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Applying the first law of. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Learn how to calculate the net work and heat in a cyclic process. Change In Internal Energy For Cyclic Process.
From www.doubtnut.com
Figure shows the variation of internal energy (U) with the pressure (P Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Hence, the internal energy change in any cyclic process is zero. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. When a system undergoes a cyclic process, its initial and final internal energies are equal. Calculate physical quantities, such as the heat transferred, work. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Chemistry 231 PowerPoint Presentation, free download ID1590157 Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Hence, the internal energy change in any cyclic process is zero. When a system undergoes a cyclic process, its initial and final internal energies are equal. Understand the concepts of system, surroundings, state function,. Isothermal expansion, isochoric. Change In Internal Energy For Cyclic Process.
From mavink.com
Cyclic Process Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Isothermal expansion, isochoric removal of heat,. Understand the concepts of system, surroundings, state function,. When a system undergoes a cyclic process, its initial and final internal energies are equal. Calculate physical quantities, such as the heat transferred,. Change In Internal Energy For Cyclic Process.
From chemistnotes.com
Thermodynamic process Isothermal, Isobaric, Isochoric, Adiabatic, and Cyclic Process Change In Internal Energy For Cyclic Process Learn how to calculate the net work and heat in a cyclic process involving four steps: Hence, the internal energy change in any cyclic process is zero. Understand the concepts of system, surroundings, state function,. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Isothermal expansion, isochoric removal of heat,. Calculate physical. Change In Internal Energy For Cyclic Process.
From www.toppr.com
In a cyclic process , the internal energy of the gas Change In Internal Energy For Cyclic Process Isothermal expansion, isochoric removal of heat,. Applying the first law of. Learn how to calculate the net work and heat in a cyclic process involving four steps: Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Understand the concepts of system, surroundings, state function,. When a system undergoes a cyclic. Change In Internal Energy For Cyclic Process.
From www.doubtnut.com
A sample of an ideal gas is taken through the cyclicprocess ABCA show Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Learn how to calculate the net work and heat in a cyclic process involving four steps: When a system undergoes a cyclic process, its initial and final internal energies are equal. Calculate physical quantities, such as the. Change In Internal Energy For Cyclic Process.
From www.youtube.com
How to Find the Work Done in Cyclic Process in Thermodynamics (Internal Energy) YouTube Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Isothermal. Change In Internal Energy For Cyclic Process.
From www.youtube.com
Change in Internal energy in processes, Cyclic process YouTube Change In Internal Energy For Cyclic Process Hence, the internal energy change in any cyclic process is zero. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Learn how to calculate the net work and heat in a cyclic process involving four steps: Understand the concepts of system, surroundings, state function,. Additionally, the change in internal energy over a. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 Change In Internal Energy For Cyclic Process When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate the net work and heat in a cyclic process involving four steps: Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Understand the concepts of system, surroundings,. Change In Internal Energy For Cyclic Process.
From www.doubtnut.com
An ideal gas γ= 1.67 is taken through the process abc shown in fig Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Isothermal expansion, isochoric removal of heat,. When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate the net work and heat in a cyclic process involving four steps: Additionally, the change in internal energy over a complete cycle is zero, indicating that the. Change In Internal Energy For Cyclic Process.
From jamar-kguzman.blogspot.com
An Ideal Gas Is Made to Undergo the Cyclic Process Change In Internal Energy For Cyclic Process Hence, the internal energy change in any cyclic process is zero. Understand the concepts of system, surroundings, state function,. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Learn how to calculate the net work and heat in a cyclic process involving four steps: Learn how. Change In Internal Energy For Cyclic Process.
From www.youtube.com
Find work done change in internal energy and the heat added to the gas in a cyclic process 134 Change In Internal Energy For Cyclic Process Hence, the internal energy change in any cyclic process is zero. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Calculate physical quantities, such as the heat transferred, work. Change In Internal Energy For Cyclic Process.
From www.researchgate.net
Change of internal energy and entropy in conventional (curve 1) and... Download Scientific Diagram Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Applying the first law of. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. When a system undergoes a cyclic process, its initial and final internal energies are equal. Hence, the internal energy change in any cyclic process is zero. Learn how. Change In Internal Energy For Cyclic Process.
From www.numerade.com
SOLVED thermodynamic system undergoes cyclic process as shown in the figure The cycle consists Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Hence, the internal energy change in any cyclic process is zero. Isothermal expansion, isochoric removal of heat,. When. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download ID3206933 Change In Internal Energy For Cyclic Process Applying the first law of. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Understand the concepts of system, surroundings, state function,. Isothermal expansion, isochoric removal of heat,. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Learn how to calculate the. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Thermodynamic Processes PowerPoint Presentation, free download ID6965910 Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Isothermal expansion, isochoric removal of heat,. When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate changes in internal energy and the flow of. Change In Internal Energy For Cyclic Process.
From www.thebigger.com
Explain Cyclic Process with example. Change In Internal Energy For Cyclic Process When a system undergoes a cyclic process, its initial and final internal energies are equal. Learn how to calculate the net work and heat in a cyclic process involving four steps: Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Additionally, the change in internal energy over a complete cycle. Change In Internal Energy For Cyclic Process.
From www.youtube.com
Assertion (A) Internal energy change in a cyclic process is zero. Reason (R ) Internal energy Change In Internal Energy For Cyclic Process When a system undergoes a cyclic process, its initial and final internal energies are equal. Hence, the internal energy change in any cyclic process is zero. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Calculate physical quantities, such as the heat transferred, work done, and. Change In Internal Energy For Cyclic Process.
From kunduz.com
[ANSWERED] Figure shows a cyclic process Heat absorbed in process A B Kunduz Change In Internal Energy For Cyclic Process Learn how to calculate the net work and heat in a cyclic process involving four steps: Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Applying the first law of. When a system undergoes a cyclic process, its initial and final internal energies are equal. Isothermal. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Honors Physics PowerPoint Presentation, free download ID3195016 Change In Internal Energy For Cyclic Process Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Learn how to calculate the net work and heat in a cyclic process involving four steps: Hence, the internal energy change in any cyclic process is zero. Additionally, the change in internal energy over a complete cycle is zero, indicating that. Change In Internal Energy For Cyclic Process.
From www.youtube.com
Thermodynamics Calculate the change in internal energy YouTube Change In Internal Energy For Cyclic Process Understand the concepts of system, surroundings, state function,. Learn how to calculate the net work and heat in a cyclic process involving four steps: Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Applying the first law of. Learn how to calculate changes in internal energy and the flow of. Change In Internal Energy For Cyclic Process.
From www.youtube.com
CHEM 101 Change in Internal Energy, Heat, and PressureVolume Work 2 YouTube Change In Internal Energy For Cyclic Process Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Isothermal expansion, isochoric removal of heat,. Hence, the internal energy change in any cyclic process is zero. Applying. Change In Internal Energy For Cyclic Process.
From www.youtube.com
Find work done change in internal energy and the heat added to the gas in a cyclic process 133 Change In Internal Energy For Cyclic Process Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Additionally, the change in internal energy over a complete cycle is zero, indicating that the total heat transfer equals the total work done. When a system undergoes a cyclic process, its initial and final internal energies are equal. Hence, the internal energy change. Change In Internal Energy For Cyclic Process.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID1607569 Change In Internal Energy For Cyclic Process Hence, the internal energy change in any cyclic process is zero. Isothermal expansion, isochoric removal of heat,. Understand the concepts of system, surroundings, state function,. Calculate physical quantities, such as the heat transferred, work done, and internal energy change for isothermal, adiabatic, and cyclical. Applying the first law of. Learn how to calculate the net work and heat in a. Change In Internal Energy For Cyclic Process.
From brainly.com
A gas is taken through the cyclic process described in the figure. (The x axis is marked in Change In Internal Energy For Cyclic Process Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Understand the concepts of system, surroundings, state function,. Hence, the internal energy change in any cyclic process is zero. Applying the first law of. Isothermal expansion, isochoric removal of heat,. When a system undergoes a cyclic process, its initial and final internal energies. Change In Internal Energy For Cyclic Process.