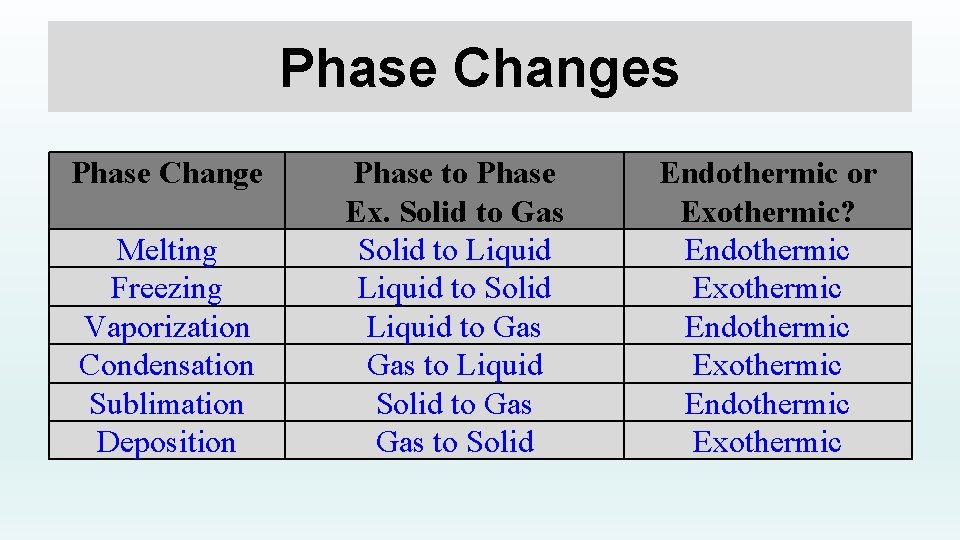
Evaporation Condensation Sublimation Deposition Freezing Melting . When moving from a more ordered state to a less ordered state, energy input is required. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Here is how you would classify the phase changes as endothermic or exothermic: Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Melting is the conversion of a solid to a liquid. We witness and utilize changes of physical state, or phase transitions, in a great number of ways. When a solid is converted directly to a gas, the process is known as sublimation. As one example of global significance, consider the evaporation, condensation,.
from slidetodoc.com
Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. When a solid is converted directly to a gas, the process is known as sublimation. As one example of global significance, consider the evaporation, condensation,. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. When moving from a more ordered state to a less ordered state, energy input is required. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Here is how you would classify the phase changes as endothermic or exothermic:
Changes of State Melting Freezing Vaporization Evaporation Condensation
Evaporation Condensation Sublimation Deposition Freezing Melting When moving from a more ordered state to a less ordered state, energy input is required. As one example of global significance, consider the evaporation, condensation,. When moving from a more ordered state to a less ordered state, energy input is required. Melting is the conversion of a solid to a liquid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Here is how you would classify the phase changes as endothermic or exothermic: We witness and utilize changes of physical state, or phase transitions, in a great number of ways. When a solid is converted directly to a gas, the process is known as sublimation. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation.
From slideplayer.com
Changes of State Melting, Freezing, Vaporization, Evaporation, Condensation, Sublimation Evaporation Condensation Sublimation Deposition Freezing Melting When moving from a more ordered state to a less ordered state, energy input is required. Melting is the conversion of a solid to a liquid. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. When a solid is converted directly to a gas, the process is known. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.numerade.com
SOLVED matter. Condensation Deposition Evaporation Freezing Melting Sublimation Q1. Which Evaporation Condensation Sublimation Deposition Freezing Melting Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Here is how you would classify the phase changes as endothermic or exothermic: Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When a solid is converted directly to a gas, the process is known as sublimation. Define melting, freezing, vaporization,. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
Changing states of matter 🔁 Melting, Freezing, Evaporation, Condensation, Deposition Evaporation Condensation Sublimation Deposition Freezing Melting The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Here is how you would classify the phase changes as endothermic or exothermic: Melting is the conversion of a solid to a liquid. When moving from a more ordered state to a less ordered state, energy input is required. We witness and. Evaporation Condensation Sublimation Deposition Freezing Melting.
From pdfslide.net
(PDF) 6. What is evaporation? · Freezing, melting, boiling Evaporation Condensation Sublimation Deposition Freezing Melting Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Melting is the conversion. Evaporation Condensation Sublimation Deposition Freezing Melting.
From stock.adobe.com
Physical states of matter.Solid, liquid and gas.Melting, freezing evaporation, condensation Evaporation Condensation Sublimation Deposition Freezing Melting Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. As one example of global significance, consider the evaporation, condensation,. Here is how you would classify the phase changes as endothermic or exothermic: Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
Change in States of matter Freezing Condensation Melting Evaporation by Heating & Cooling Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Here is how you would classify the phase changes as endothermic or exothermic: Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. When a solid is converted directly to. Evaporation Condensation Sublimation Deposition Freezing Melting.
From pdfslide.net
(PDF) 6. What is evaporation? · Freezing, melting, boiling Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When moving from a more ordered state to a less ordered state, energy input is required. Here is how you would classify the phase changes as endothermic or exothermic: Understand various stages of phase change. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slidetodoc.com
Changes of State Melting Freezing Vaporization Evaporation Condensation Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. When moving from a more ordered state to a less ordered state, energy input is required. When a solid is converted directly to a gas, the process is known as sublimation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Here is how you would classify the phase changes as endothermic. Evaporation Condensation Sublimation Deposition Freezing Melting.
From vdocuments.mx
Phase changes Melting Freezing Vaporization (or evaporation) Condensation Sublimation Deposition Evaporation Condensation Sublimation Deposition Freezing Melting Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. When a solid is converted directly. Evaporation Condensation Sublimation Deposition Freezing Melting.
From study.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation & Deposition Video Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. As one example of global significance, consider the evaporation, condensation,. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid.. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slidetodoc.com
Changes of State Melting Freezing Vaporization Evaporation Condensation Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Here is how you would classify the phase changes as endothermic or exothermic: Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Melting is the conversion of a solid. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slideplayer.com
Chapter 18 Characteristics of water… Frozen water = ice ppt download Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. As one example of global significance, consider the evaporation, condensation,. Energy changes in sublimation/deposition are similar to melting/freezing but. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slidetodoc.com
Changes of State Melting Freezing Vaporization Evaporation Condensation Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. As one example of global significance, consider the evaporation, condensation,. When moving from a more ordered state. Evaporation Condensation Sublimation Deposition Freezing Melting.
From blogdejuanmateacher.blogspot.com
El blog de juanmateacher 6º CHANGES OF STATE OF MATTER Evaporation Condensation Sublimation Deposition Freezing Melting Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. When moving from a more ordered state to a less ordered state, energy input is required. When a solid is converted directly to a gas, the process is known as sublimation. As one example of global significance, consider the evaporation, condensation,. Here is how you. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.numerade.com
Determine whether heating or cooling takes place during each process. evaporation condensation Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. As one example of global significance, consider the evaporation, condensation,. When moving from a more ordered state to a less ordered state, energy input is required. The reverse of sublimation is called deposition, a process. Evaporation Condensation Sublimation Deposition Freezing Melting.
From studiousguy.com
7 Sublimation Examples in Daily Life StudiousGuy Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. As one example of global significance, consider the evaporation, condensation,. When a solid is converted directly to. Evaporation Condensation Sublimation Deposition Freezing Melting.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Energy changes in sublimation/deposition are similar to melting/freezing but happen. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slidetodoc.com
Changes of State Melting Freezing Vaporization Evaporation Condensation Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. When a solid is converted directly to a gas, the process is known as sublimation. When moving from a more ordered state to a less ordered state, energy input is required. Melting is the conversion of a solid to a liquid. The reverse. Evaporation Condensation Sublimation Deposition Freezing Melting.
From app.emaze.com
on emaze Evaporation Condensation Sublimation Deposition Freezing Melting Here is how you would classify the phase changes as endothermic or exothermic: When moving from a more ordered state to a less ordered state, energy input is required. As one example of global significance, consider the evaporation, condensation,. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Melting is the. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.numerade.com
SOLVED solid liquid gas critical point triple point evaporation condensation above phase d Evaporation Condensation Sublimation Deposition Freezing Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When moving from a more ordered state to a less ordered state, energy input is required. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. We witness and. Evaporation Condensation Sublimation Deposition Freezing Melting.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Evaporation Condensation Sublimation Deposition Freezing Melting Here is how you would classify the phase changes as endothermic or exothermic: When moving from a more ordered state to a less ordered state, energy input is required. We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.numerade.com
SOLVED 'Table 2 phase change freezing melting evaporation condensation sublimation deposition Evaporation Condensation Sublimation Deposition Freezing Melting Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When moving from a more ordered state to a less ordered state, energy input is required. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. We. Evaporation Condensation Sublimation Deposition Freezing Melting.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation, condensation, sublimation Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Here is how you would classify the phase changes as endothermic or exothermic: Melting is the conversion of a solid to a liquid. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Melting, evaporation and sublimation are endothermic processes. Evaporation Condensation Sublimation Deposition Freezing Melting.
From slideplayer.com
Chapter 10 Theory “Moving”) ppt download Evaporation Condensation Sublimation Deposition Freezing Melting As one example of global significance, consider the evaporation, condensation,. Here is how you would classify the phase changes as endothermic or exothermic: When a solid is converted directly to a gas, the process is known as sublimation. Melting is the conversion of a solid to a liquid. Understand various stages of phase change such as deposition, sublimation, condensation &. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
Science 4 Changes of Materials Freezing, Melting, Condensation, Evaporation, Sublimation YouTube Evaporation Condensation Sublimation Deposition Freezing Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. When a solid is converted directly. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.pinterest.co.kr
States of Water infographic diagram including gas liquid and solid also showing all processes Evaporation Condensation Sublimation Deposition Freezing Melting We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Define melting, freezing, vaporization, condensation, sublimation, and deposition. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Here is how you would classify the phase changes as endothermic or exothermic: When moving from a. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
FREEZING, MELTING, EVAPORATION, CONDENSATION, SUBLIMATION, DEPOSITION / Science for kids YouTube Evaporation Condensation Sublimation Deposition Freezing Melting When a solid is converted directly to a gas, the process is known as sublimation. Melting is the conversion of a solid to a liquid. When moving from a more ordered state to a less ordered state, energy input is required. As one example of global significance, consider the evaporation, condensation,. Understand various stages of phase change such as deposition,. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
Change of State Evaporation Condensation Melting Freezing Sublimation Deposition Evaporation Condensation Sublimation Deposition Freezing Melting As one example of global significance, consider the evaporation, condensation,. Here is how you would classify the phase changes as endothermic or exothermic: Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Melting is the conversion of a solid to a liquid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. When moving from a. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.numerade.com
SOLVED Question 35 (2 points) Which pair of processes are both Exothermic. Sublimation and Evaporation Condensation Sublimation Deposition Freezing Melting When moving from a more ordered state to a less ordered state, energy input is required. Define melting, freezing, vaporization, condensation, sublimation, and deposition. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. As one example of global significance, consider the evaporation, condensation,. Melting is the conversion of a solid to. Evaporation Condensation Sublimation Deposition Freezing Melting.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter with different molecular Evaporation Condensation Sublimation Deposition Freezing Melting Melting is the conversion of a solid to a liquid. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When moving from a more ordered state to a less ordered state,. Evaporation Condensation Sublimation Deposition Freezing Melting.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Evaporation Condensation Sublimation Deposition Freezing Melting Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Here is how you would classify the phase changes as endothermic or exothermic: We witness and utilize changes of physical state, or phase transitions, in a great number of ways. Energy changes. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.youtube.com
Phase transitionsStates of matterMeltingvaporizationDepositionSublimationChemistry grade Evaporation Condensation Sublimation Deposition Freezing Melting The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Melting is the conversion of a solid to a liquid. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. When moving from a more ordered state to a less ordered state, energy input is required. Energy changes in. Evaporation Condensation Sublimation Deposition Freezing Melting.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Evaporation Condensation Sublimation Deposition Freezing Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly into the solid. Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. When moving from a more ordered state to a less ordered state,. Evaporation Condensation Sublimation Deposition Freezing Melting.
From stock.adobe.com
Change in state with gas, liquid and solid water outline diagram. Labeled educational scheme Evaporation Condensation Sublimation Deposition Freezing Melting Melting, evaporation and sublimation are endothermic processes while freezing, condensation and deposition are exothermic processes. Here is how you would classify the phase changes as endothermic or exothermic: As one example of global significance, consider the evaporation, condensation,. When a solid is converted directly to a gas, the process is known as sublimation. We witness and utilize changes of physical. Evaporation Condensation Sublimation Deposition Freezing Melting.
From www.pinterest.com
States of Matter Unit Melting, Freezing, Evaporation, Condensation, Sublimation, Deposition Evaporation Condensation Sublimation Deposition Freezing Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. When a solid is converted directly to a gas, the process is known as sublimation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Understand various stages of phase change such as deposition, sublimation, condensation & evaporation. When moving from a more ordered state to a. Evaporation Condensation Sublimation Deposition Freezing Melting.