Metal + Water Equation . Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. Metals form respective metal hydroxide and hydrogen gas when react with water. When you wet a piece of sodium metal, it gets hot and fizzes; Metal + water ⇨ metal hydroxide + hydrogen; In each case, a solution of the metal hydroxide is produced. All of these metals react vigorously or even explosively with cold water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water.
from www.numerade.com
In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In each case, a solution of the metal hydroxide is produced. Metals form respective metal hydroxide and hydrogen gas when react with water. Metal + water ⇨ metal hydroxide + hydrogen; In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. All of these metals react vigorously or even explosively with cold water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from.
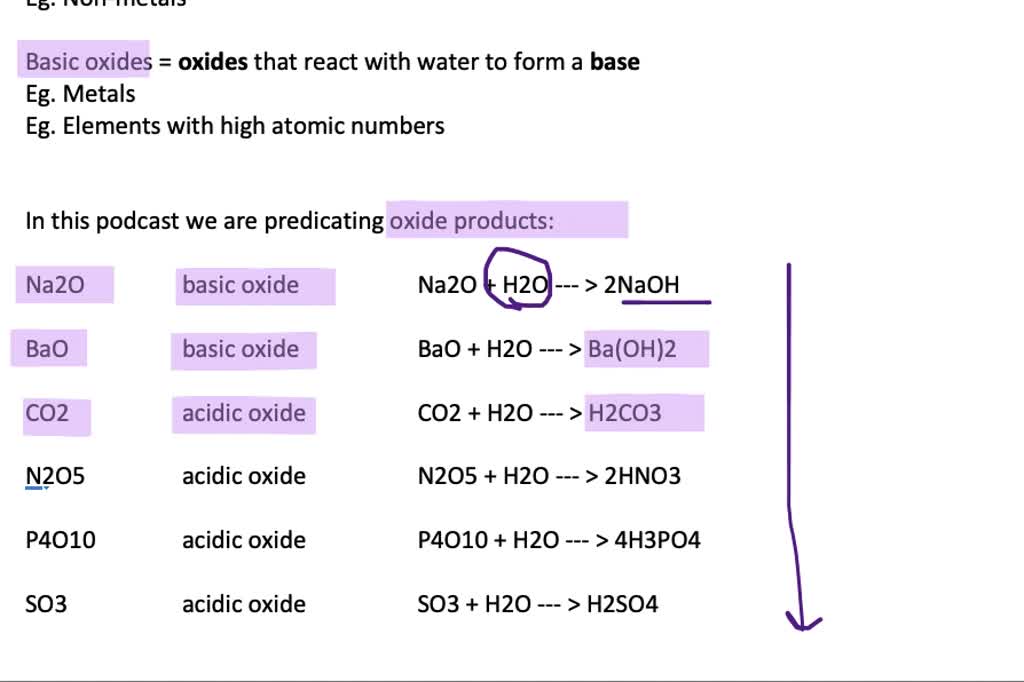
Metal oxides can react with water to form bases.
Metal + Water Equation Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of these metals react vigorously or even explosively with cold water. Metals form respective metal hydroxide and hydrogen gas when react with water. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. Metal + water ⇨ metal hydroxide + hydrogen; If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. When you wet a piece of sodium metal, it gets hot and fizzes; In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. In each case, a solution of the metal hydroxide is produced.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Metal + Water Equation When you wet a piece of sodium metal, it gets hot and fizzes; Metals form respective metal hydroxide and hydrogen gas when react with water. In each case, a solution of the metal hydroxide is produced. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as. Metal + Water Equation.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID5525228 Metal + Water Equation Metal + water ⇨ metal hydroxide + hydrogen; All of these metals react vigorously or even explosively with cold water. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and. Metal + Water Equation.
From www.numerade.com
SOLVED Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to Metal + Water Equation When you wet a piece of sodium metal, it gets hot and fizzes; Metals form respective metal hydroxide and hydrogen gas when react with water. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Metal + water ⇨ metal hydroxide + hydrogen; In this tutorial, you will learn about the. Metal + Water Equation.
From www.vecteezy.com
Process of rusting chemical equation 1868434 Vector Art at Vecteezy Metal + Water Equation Metals form respective metal hydroxide and hydrogen gas when react with water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. When you wet a piece of sodium metal, it gets hot and fizzes; Recap the reactivity series of metals and how it's used to. Metal + Water Equation.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)翰林国际教育 Metal + Water Equation All of these metals react vigorously or even explosively with cold water. When you wet a piece of sodium metal, it gets hot and fizzes; In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. Metals form respective metal hydroxide and hydrogen gas when react with water. In this event,. Metal + Water Equation.
From sciencenotes.org
Sodium in Water Chemistry Demonstration Metal + Water Equation Metal + water ⇨ metal hydroxide + hydrogen; In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. Recap the reactivity series of metals and how it's used to. Metal + Water Equation.
From www.vrogue.co
Group 2 Metal Reactions A Level Chemistrystudent vrogue.co Metal + Water Equation All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Metal + water ⇨ metal hydroxide + hydrogen; In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. When you wet a piece of sodium metal, it gets hot and fizzes; Metals. Metal + Water Equation.
From ppt-online.org
Metals презентация онлайн Metal + Water Equation If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. Metals form respective metal hydroxide and hydrogen gas when react with water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. In this tutorial,. Metal + Water Equation.
From mstimms-year9science.blogspot.com
Year 9 science November 2012 Metal + Water Equation Metals form respective metal hydroxide and hydrogen gas when react with water. When you wet a piece of sodium metal, it gets hot and fizzes; Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. In each case, a solution of the metal hydroxide is produced. All of these metals react. Metal + Water Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Metal + Water Equation If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. Metals form respective metal hydroxide and hydrogen gas when react with water. Learn how group. Metal + Water Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Metal + Water Equation Metal + water ⇨ metal hydroxide + hydrogen; Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of these metals react vigorously or. Metal + Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Metal + Water Equation All of these metals react vigorously or even explosively with cold water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In each case, a. Metal + Water Equation.
From winnerseducation.com
Reaction of Metals with water, steam and dilute acid (1) Winners Education Centre The Metal + Water Equation All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Metal + water ⇨ metal hydroxide + hydrogen; When you wet a piece of sodium metal, it gets hot and fizzes; If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from.. Metal + Water Equation.
From www.thesciencehive.co.uk
Transition Elements* — the science sauce Metal + Water Equation In each case, a solution of the metal hydroxide is produced. When you wet a piece of sodium metal, it gets hot and fizzes; If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. Metal + water ⇨ metal hydroxide + hydrogen; All of group 1 elements—lithium, sodium, potassium,. Metal + Water Equation.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID4271268 Metal + Water Equation Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. Metal + water ⇨ metal hydroxide + hydrogen; When you wet a piece. Metal + Water Equation.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free download ID5525447 Metal + Water Equation All of these metals react vigorously or even explosively with cold water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. In each case, a solution of the metal hydroxide is produced. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react. Metal + Water Equation.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal + Water Equation In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. In each case, a solution of the metal hydroxide is produced. All of these metals react vigorously or even explosively with cold water. Metal + water ⇨ metal hydroxide + hydrogen; If a metal is above hydrogen in the reactivity. Metal + Water Equation.
From www.numerade.com
Metal oxides can react with water to form bases. Metal + Water Equation Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Metal + water ⇨ metal hydroxide + hydrogen; In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. In this. Metal + Water Equation.
From www.youtube.com
Reactions Of Metals With Water Reactions Chemistry FuseSchool YouTube Metal + Water Equation Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Metals form respective metal hydroxide and hydrogen gas when react with water. In each case, a solution of the metal hydroxide is produced. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air,. Metal + Water Equation.
From www.thesciencehive.co.uk
Group 2* — the science sauce Metal + Water Equation Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. In each case, a solution of the metal hydroxide is produced. In this event, the group 1 metal is oxidized. Metal + Water Equation.
From www.nagwa.com
Question Video Identifying the Balanced Chemical Equation for the Reaction of Sodium Metal and Metal + Water Equation All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Metal + water ⇨ metal hydroxide + hydrogen; All of these metals react vigorously or even explosively with cold water. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Metals form respective. Metal + Water Equation.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free download ID3099937 Metal + Water Equation Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. All of these metals react vigorously or even explosively with cold water. In this tutorial, you will learn about the reaction between sodium and water, what causes it and how it works. In this event, the group 1 metal is oxidized. Metal + Water Equation.
From www.nagwa.com
Question Video Identifying the Chemical Equation That Describes the Reaction of Scandium and Metal + Water Equation Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Metals form respective metal hydroxide and hydrogen gas when react with water. Metal + water ⇨ metal hydroxide + hydrogen; Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam. Metal + Water Equation.
From slidetodoc.com
IGCSE Chemistry Metals and Metal Compounds 1 of Metal + Water Equation Metals form respective metal hydroxide and hydrogen gas when react with water. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In each case, a solution of the metal hydroxide is produced. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water. Metal + Water Equation.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free download ID6645971 Metal + Water Equation Metals form respective metal hydroxide and hydrogen gas when react with water. All of these metals react vigorously or even explosively with cold water. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. Metal + water ⇨ metal hydroxide + hydrogen; Recap the reactivity series of metals and how it's. Metal + Water Equation.
From wordwall.net
Metals + Water Equations Missing word Metal + Water Equation In each case, a solution of the metal hydroxide is produced. Metals form respective metal hydroxide and hydrogen gas when react with water. Metal + water ⇨ metal hydroxide + hydrogen; When you wet a piece of sodium metal, it gets hot and fizzes; All of these metals react vigorously or even explosively with cold water. Learn how group 1. Metal + Water Equation.
From brainly.in
In what forms are metals found in nature? With the help of examples, explain how metals react Metal + Water Equation All of these metals react vigorously or even explosively with cold water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Metals form respective metal. Metal + Water Equation.
From ppt-online.org
The alkali metals презентация онлайн Metal + Water Equation Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of these metals react vigorously or even explosively with cold water. When you wet a piece of sodium metal, it gets hot and fizzes; All of group 1 elements—lithium, sodium, potassium, rubidium and. Metal + Water Equation.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID4271268 Metal + Water Equation All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Learn how group 1 elements, also known as alkali metals, react with water to form metal hydroxides and hydrogen. When you wet a piece of sodium metal, it gets hot and fizzes; In this tutorial, you will learn about the reaction between. Metal + Water Equation.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Metal + Water Equation All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Metals form respective metal hydroxide and hydrogen gas when react with water. Metal + water ⇨ metal hydroxide + hydrogen; When you wet a piece of sodium metal, it gets hot and fizzes; In this tutorial, you will learn about the reaction. Metal + Water Equation.
From www.doubtnut.com
Apparatus Beakers. Chemicals Samples of various metals , water. Metal + Water Equation In each case, a solution of the metal hydroxide is produced. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In this tutorial, you will. Metal + Water Equation.
From www.numerade.com
SOLVED When potassium metal is placed in water, a large amount of energy is released as Metal + Water Equation If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from. When you wet a piece of sodium metal, it gets hot and fizzes; Metals form respective metal hydroxide and hydrogen gas when react with water. Recap the reactivity series of metals and how it's used to predict the outcomes. Metal + Water Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Resources Metal + Water Equation All of these metals react vigorously or even explosively with cold water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. When you wet a piece of sodium metal, it gets hot and fizzes; In each case, a solution of the metal hydroxide is produced.. Metal + Water Equation.
From general.chemistrysteps.com
Predicting The Products of Chemical Reactions Metal + Water Equation All of these metals react vigorously or even explosively with cold water. Metal + water ⇨ metal hydroxide + hydrogen; Metals form respective metal hydroxide and hydrogen gas when react with water. In each case, a solution of the metal hydroxide is produced. If a metal is above hydrogen in the reactivity series it should, in principle, be able to. Metal + Water Equation.
From workerdecline11.gitlab.io
Outrageous How Lithium Reacts With Cold Water Physics Formula Class 11 And 12 Metal + Water Equation All of these metals react vigorously or even explosively with cold water. In this event, the group 1 metal is oxidized to its metal ion and water is reduced to form hydrogen gas and hydroxide ions. All of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Recap the reactivity series of metals. Metal + Water Equation.