What Is Boiling Point Example . Therefore, the boiling point of a liquid depends on. The change from a liquid phase to. Learn what boiling point is and how it depends on external pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. This is the boiling point. See examples of boiling point at different locations and conditions, such as space. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. See how the boiling point of water decreases as altitude increases, with. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water.
from www.vedantu.com
If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn what boiling point is and how it depends on external pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. See how the boiling point of water decreases as altitude increases, with. This is the boiling point. See examples of boiling point at different locations and conditions, such as space. Therefore, the boiling point of a liquid depends on. The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water.
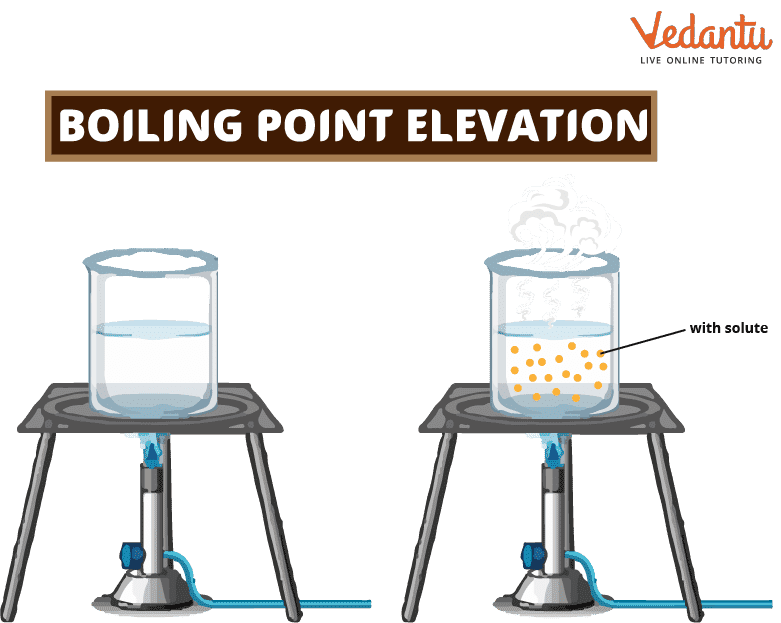
Boiling Point Elevation Learn Important Terms and Concepts
What Is Boiling Point Example The change from a liquid phase to. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. See how the boiling point of water decreases as altitude increases, with. This is the boiling point. The change from a liquid phase to. See examples of boiling point at different locations and conditions, such as space. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Therefore, the boiling point of a liquid depends on. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Learn what boiling point is and how it depends on external pressure.
From www.youtube.com
Boiling Point from Heat of Vaporization (Example) YouTube What Is Boiling Point Example If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn what boiling point is and how it depends on external pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.. What Is Boiling Point Example.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free download ID2792222 What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal. What Is Boiling Point Example.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. See how the boiling point of water decreases as altitude increases, with. Therefore, the boiling point of a liquid depends on. See examples of boiling point at different locations and conditions, such as space. This is the boiling point. Learn what boiling point is and how it. What Is Boiling Point Example.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. This is the boiling point. Learn what boiling point is and how it depends on external pressure. See how the boiling point of water decreases as altitude increases, with. The change from a liquid phase to. Boiling is the process by which a liquid turns into a. What Is Boiling Point Example.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free download ID2808167 What Is Boiling Point Example This is the boiling point. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the. What Is Boiling Point Example.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Vector Adobe Stock What Is Boiling Point Example The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. See how the boiling point of water decreases as altitude increases, with. This is the boiling point. The change from a liquid phase to. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is. What Is Boiling Point Example.
From www.youtube.com
CHEM 201 Calculating Boiling Point of a NonElectrolyte Solution YouTube What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. This is the boiling point. See examples of boiling point at different locations and conditions, such as space. Learn what boiling point is and how. What Is Boiling Point Example.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Is Boiling Point Example This is the boiling point. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred. What Is Boiling Point Example.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Learn what boiling point is and how it depends on external pressure. See examples of boiling point at different locations and conditions, such as space. This is the boiling point. See how the boiling point of water decreases as altitude increases, with. If this pressure is the. What Is Boiling Point Example.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is Boiling Point Example See how the boiling point of water decreases as altitude increases, with. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This is the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to. What Is Boiling Point Example.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 What Is Boiling Point Example If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. See how the boiling point of water decreases as altitude increases, with. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when. What Is Boiling Point Example.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Boiling Point Example The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. Learn what boiling point is and how it depends on external pressure. See examples of boiling point at different locations and conditions, such as space. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. If this pressure is the standard. What Is Boiling Point Example.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is Boiling Point Example See how the boiling point of water decreases as altitude increases, with. The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. This is the boiling point. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn what boiling point is and how. What Is Boiling Point Example.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn what boiling point is and how it depends on external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the. What Is Boiling Point Example.
From www.youtube.com
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Point Relationship What Is Boiling Point Example This is the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. See examples of boiling point at different locations and conditions, such as space. The boiling point is the temperature at which the vapor pressure of a liquid. What Is Boiling Point Example.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is Boiling Point Example The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn what boiling point is and how it depends on external pressure. The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. This is the boiling point. Boiling point, temperature at which the. What Is Boiling Point Example.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Vector Illustration of What Is Boiling Point Example If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. This is the boiling point. Therefore, the boiling point of a liquid depends on. See examples of boiling point. What Is Boiling Point Example.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID6431061 What Is Boiling Point Example Learn what boiling point is and how it depends on external pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The change from a liquid phase to. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Therefore, the boiling point of a. What Is Boiling Point Example.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID6431061 What Is Boiling Point Example The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. This is the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. What Is Boiling Point Example.
From jsmithmoore.com
Boiling point of ethanol celsius What Is Boiling Point Example This is the boiling point. See how the boiling point of water decreases as altitude increases, with. See examples of boiling point at different locations and conditions, such as space. Therefore, the boiling point of a liquid depends on. The change from a liquid phase to. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Boiling Point Example.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point Formula Boiling Points YouTube What Is Boiling Point Example Therefore, the boiling point of a liquid depends on. See examples of boiling point at different locations and conditions, such as space. The change from a liquid phase to. Learn what boiling point is and how it depends on external pressure. See how the boiling point of water decreases as altitude increases, with. Learn how atmospheric pressure, elevation, and impurities. What Is Boiling Point Example.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is Boiling Point Example Learn what boiling point is and how it depends on external pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. See examples of boiling point at different locations and conditions, such as space. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Boiling Point Example.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is Boiling Point Example Therefore, the boiling point of a liquid depends on. This is the boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn how atmospheric. What Is Boiling Point Example.
From www.slideserve.com
PPT Flash PointIgnition Point PowerPoint Presentation ID310797 What Is Boiling Point Example Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Learn what boiling point is and how it depends on external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Boiling is the process by which a. What Is Boiling Point Example.
From www.slideserve.com
PPT States, Boiling Point, Melting Point, and Solubility PowerPoint Presentation ID6867774 What Is Boiling Point Example If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. See examples of boiling point at different locations and conditions, such as space. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. What Is Boiling Point Example.
From www.youtube.com
Boiling Point from PVT Diagram (Example) YouTube What Is Boiling Point Example Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. See how the boiling point of water decreases as altitude increases, with. The change from a liquid phase to. Learn what boiling point is and how it depends on external pressure. See examples of boiling point at different. What Is Boiling Point Example.
From www.slideserve.com
PPT melting & boiling points PowerPoint Presentation, free download ID1990444 What Is Boiling Point Example The change from a liquid phase to. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Therefore, the boiling point of a liquid depends on. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Learn what boiling. What Is Boiling Point Example.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is Boiling Point Example See examples of boiling point at different locations and conditions, such as space. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Learn how atmospheric pressure,. What Is Boiling Point Example.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Boiling Point Example Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. See examples of boiling point at different locations and conditions, such as space. See how the boiling point of water decreases as altitude increases, with. This is the boiling point. Boiling is the process by which a liquid. What Is Boiling Point Example.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is Boiling Point Example Therefore, the boiling point of a liquid depends on. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at. What Is Boiling Point Example.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free download ID6814627 What Is Boiling Point Example This is the boiling point. Therefore, the boiling point of a liquid depends on. See examples of boiling point at different locations and conditions, such as space. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Learn how atmospheric pressure, elevation, and impurities affect the boiling point. What Is Boiling Point Example.
From www.worksheetsplanet.com
What is Boiling Point What Is Boiling Point Example Therefore, the boiling point of a liquid depends on. See how the boiling point of water decreases as altitude increases, with. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding. What Is Boiling Point Example.
From www.slideserve.com
PPT Physical and Chemical Properties 9/16/2014 PowerPoint Presentation ID5849998 What Is Boiling Point Example The change from a liquid phase to. Learn how atmospheric pressure, elevation, and impurities affect the boiling point of water. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. This is the boiling point. Boiling is the process by which a liquid turns into a vapor when. What Is Boiling Point Example.
From www.slideserve.com
PPT Chapter Introduction Lesson 1 Physical Properties Lesson 2 Density PowerPoint Presentation What Is Boiling Point Example Therefore, the boiling point of a liquid depends on. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Boiling is the process by which a liquid. What Is Boiling Point Example.
From www.youtube.com
Boiling Point Elevation Example YouTube What Is Boiling Point Example Learn what boiling point is and how it depends on external pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. See examples of boiling point at different locations and conditions, such as space. If this pressure is the standard pressure of 1 atm (101.3 kpa), then. What Is Boiling Point Example.