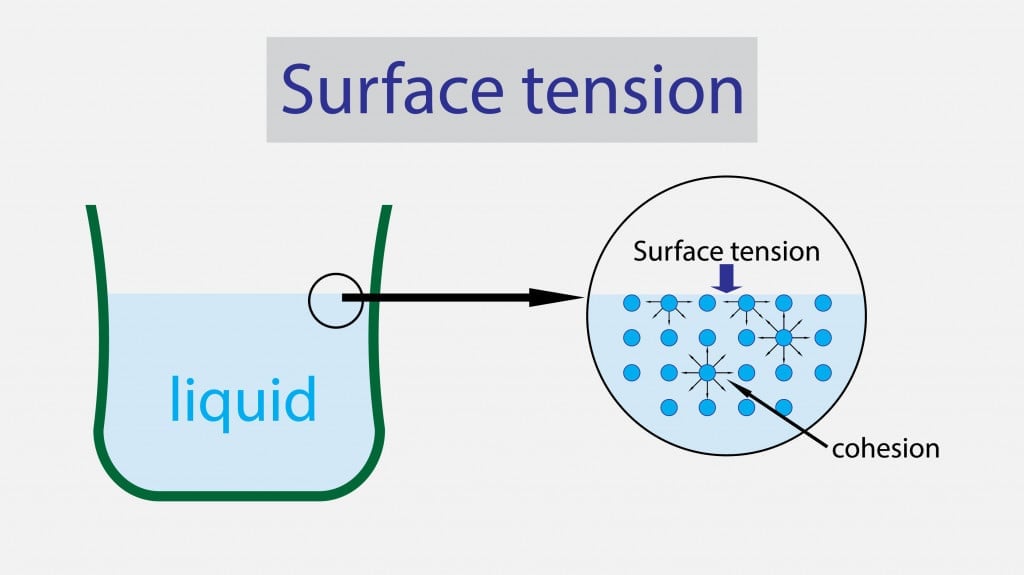
High Surface Tension Of Water Meaning . If you place a small needle on the surface of. The cohesive forces between liquid molecules. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. (a) a paper clip can “float” on. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water is one liquid known to have a very high surface tension value and is difficult to overcome. pure water, for example, has significantly high surface tension. Water has a surface tension of.
from www.scienceabc.com
pure water, for example, has significantly high surface tension. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. (a) a paper clip can “float” on. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of. If you place a small needle on the surface of. The cohesive forces between liquid molecules. water is one liquid known to have a very high surface tension value and is difficult to overcome. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them.
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC
High Surface Tension Of Water Meaning water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. pure water, for example, has significantly high surface tension. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules. Water has a surface tension of. If you place a small needle on the surface of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. (a) a paper clip can “float” on. water is one liquid known to have a very high surface tension value and is difficult to overcome.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry High Surface Tension Of Water Meaning water is one liquid known to have a very high surface tension value and is difficult to overcome. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. Water has. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download High Surface Tension Of Water Meaning If you place a small needle on the surface of. water is one liquid known to have a very high surface tension value and is difficult to overcome. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. pure water,. High Surface Tension Of Water Meaning.
From www.youtube.com
1.1 property of water HIGH surface tension YouTube High Surface Tension Of Water Meaning The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water is one liquid known to have a very high surface tension value and is difficult to overcome. If you place a small needle on. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download High Surface Tension Of Water Meaning Water has a surface tension of. pure water, for example, has significantly high surface tension. water is one liquid known to have a very high surface tension value and is difficult to overcome. If you place a small needle on the surface of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two. High Surface Tension Of Water Meaning.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts High Surface Tension Of Water Meaning water is one liquid known to have a very high surface tension value and is difficult to overcome. If you place a small needle on the surface of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to. High Surface Tension Of Water Meaning.
From www.biolinscientific.com
Surface tension of water Why is it so high? High Surface Tension Of Water Meaning surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of. The cohesive forces between liquid molecules. water, for example,. High Surface Tension Of Water Meaning.
From www.youtube.com
Surface Tension of Water Explained YouTube High Surface Tension Of Water Meaning surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download High Surface Tension Of Water Meaning The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. If you place a small needle on the surface of. Water has a surface tension of. water is one liquid known to have a very. High Surface Tension Of Water Meaning.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC High Surface Tension Of Water Meaning surface tension may be expressed, therefore, in units of energy per unit area (square metres). (a) a paper clip can “float” on. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules. High Surface Tension Of Water Meaning.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs High Surface Tension Of Water Meaning surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules. If you place a small needle on the surface of. surface tension may be expressed, therefore, in units of energy per unit area (square metres).. High Surface Tension Of Water Meaning.
From ar.inspiredpencil.com
Surface Tension Water High Surface Tension Of Water Meaning pure water, for example, has significantly high surface tension. water is one liquid known to have a very high surface tension value and is difficult to overcome. The cohesive forces between liquid molecules. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges,. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint High Surface Tension Of Water Meaning If you place a small needle on the surface of. Water has a surface tension of. water is one liquid known to have a very high surface tension value and is difficult to overcome. surface tension may be expressed, therefore, in units of energy per unit area (square metres). (a) a paper clip can “float” on. surface. High Surface Tension Of Water Meaning.
From www.narodnatribuna.info
What Is Wettability High Surface Tension Of Water Meaning (a) a paper clip can “float” on. water is one liquid known to have a very high surface tension value and is difficult to overcome. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of. High Surface Tension Of Water Meaning.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog High Surface Tension Of Water Meaning If you place a small needle on the surface of. The cohesive forces between liquid molecules. Water has a surface tension of. (a) a paper clip can “float” on. surface tension may be expressed, therefore, in units of energy per unit area (square metres). pure water, for example, has significantly high surface tension. water is one liquid. High Surface Tension Of Water Meaning.
From www.biolinscientific.com
Surface tension of water Why is it so high? High Surface Tension Of Water Meaning If you place a small needle on the surface of. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension may be expressed, therefore, in units of energy per unit area (square metres). (a) a paper clip can “float”. High Surface Tension Of Water Meaning.
From ar.inspiredpencil.com
Surface Tension Water High Surface Tension Of Water Meaning The cohesive forces between liquid molecules. pure water, for example, has significantly high surface tension. If you place a small needle on the surface of. Water has a surface tension of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen. High Surface Tension Of Water Meaning.
From www.vrogue.co
Surface Tension Definition Examples And Unit vrogue.co High Surface Tension Of Water Meaning (a) a paper clip can “float” on. The cohesive forces between liquid molecules. Water has a surface tension of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. surface. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 High Surface Tension Of Water Meaning pure water, for example, has significantly high surface tension. water is one liquid known to have a very high surface tension value and is difficult to overcome. Water has a surface tension of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges,. High Surface Tension Of Water Meaning.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog High Surface Tension Of Water Meaning Water has a surface tension of. The cohesive forces between liquid molecules. If you place a small needle on the surface of. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. pure water, for example, has significantly high surface tension.. High Surface Tension Of Water Meaning.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids High Surface Tension Of Water Meaning surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water is one liquid known to have a very high surface tension value and is difficult to overcome. surface tension may be expressed, therefore, in units of energy per unit. High Surface Tension Of Water Meaning.
From www.thoughtco.com
Surface Tension Definition in Chemistry High Surface Tension Of Water Meaning surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. If you place a small needle on the surface of. (a) a paper clip can “float”. High Surface Tension Of Water Meaning.
From exydwggxr.blob.core.windows.net
What Is A High Or Low Surface Tension at Michael Moore blog High Surface Tension Of Water Meaning water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. pure water, for example, has significantly high surface tension. The cohesive forces between liquid molecules. water is one liquid. High Surface Tension Of Water Meaning.
From giojkmyma.blob.core.windows.net
How Does Surface Tension Force Water To Curve at Linda Robles blog High Surface Tension Of Water Meaning pure water, for example, has significantly high surface tension. water is one liquid known to have a very high surface tension value and is difficult to overcome. Water has a surface tension of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a phenomenon in which the. High Surface Tension Of Water Meaning.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation High Surface Tension Of Water Meaning If you place a small needle on the surface of. Water has a surface tension of. pure water, for example, has significantly high surface tension. The cohesive forces between liquid molecules. (a) a paper clip can “float” on. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial. High Surface Tension Of Water Meaning.
From giojsbuzt.blob.core.windows.net
What Does A High Surface Tension Mean at Charles Dufresne blog High Surface Tension Of Water Meaning water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. water is one liquid known to have a very high surface tension value and is difficult to overcome. surface. High Surface Tension Of Water Meaning.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) High Surface Tension Of Water Meaning surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. (a) a paper clip can “float” on. Water has a surface tension of. pure water,. High Surface Tension Of Water Meaning.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments High Surface Tension Of Water Meaning The cohesive forces between liquid molecules. If you place a small needle on the surface of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. surface tension may be. High Surface Tension Of Water Meaning.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? High Surface Tension Of Water Meaning water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. High Surface Tension Of Water Meaning.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii High Surface Tension Of Water Meaning Water has a surface tension of. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules. pure water, for example, has significantly high surface tension. If you place a small needle on the surface of.. High Surface Tension Of Water Meaning.
From giowqghdr.blob.core.windows.net
Surface Tension Of Water Room Temperature at Margaret Dugan blog High Surface Tension Of Water Meaning If you place a small needle on the surface of. The cohesive forces between liquid molecules. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. surface tension may be. High Surface Tension Of Water Meaning.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with High Surface Tension Of Water Meaning surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water has a surface tension of. The cohesive forces between liquid molecules. surface tension may be expressed, therefore, in units of energy per unit area (square metres). pure water, for. High Surface Tension Of Water Meaning.
From giolawchz.blob.core.windows.net
Water Surface Tension Depends On at Noel Rothman blog High Surface Tension Of Water Meaning (a) a paper clip can “float” on. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water has a surface tension of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water is. High Surface Tension Of Water Meaning.
From exyqcbprj.blob.core.windows.net
Surface Tension Of Water Jet at Terry Price blog High Surface Tension Of Water Meaning water is one liquid known to have a very high surface tension value and is difficult to overcome. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. surface. High Surface Tension Of Water Meaning.
From www.youtube.com
Surface Tension YouTube High Surface Tension Of Water Meaning Water has a surface tension of. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water is one. High Surface Tension Of Water Meaning.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance High Surface Tension Of Water Meaning water is one liquid known to have a very high surface tension value and is difficult to overcome. If you place a small needle on the surface of. (a) a paper clip can “float” on. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as. High Surface Tension Of Water Meaning.