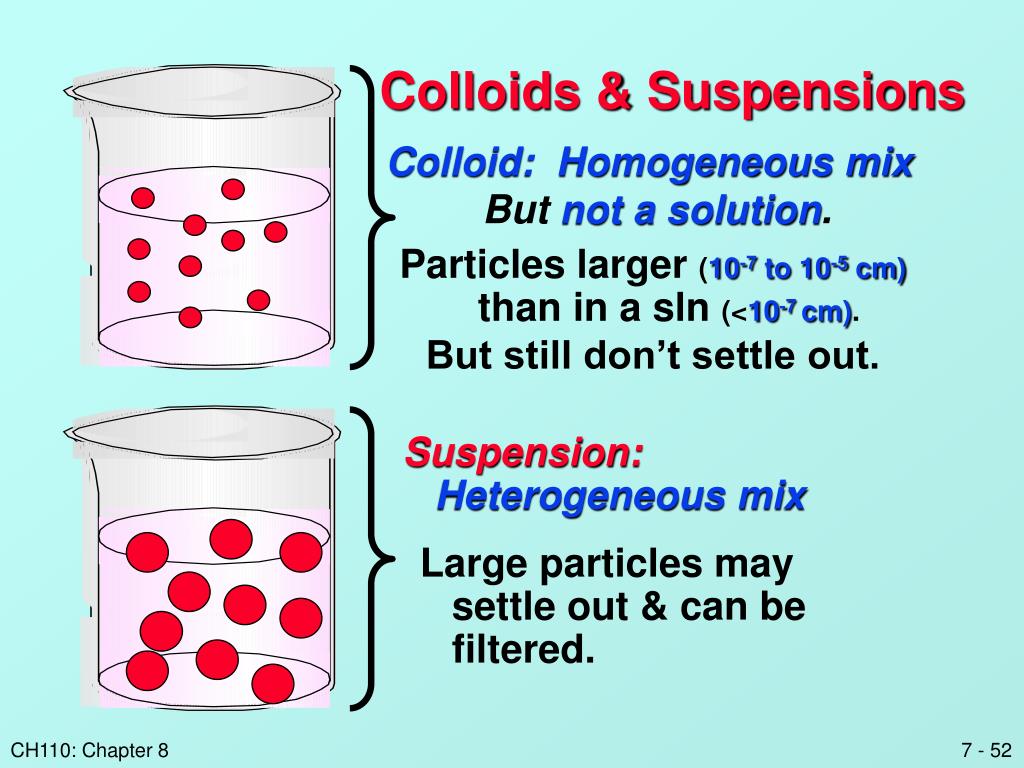
Suspension And Colloid . Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The substance that is dissolved is the solute. A solution is a homogeneous mixture of two or more components. Examples of colloids include milk, gelatin, and fog. On the other hand, a suspension is a heterogeneous mixture where solid particles. The dissolving agent is the solvent. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods.
from
Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. A solution is a homogeneous mixture of two or more components. The substance that is dissolved is the solute. The dissolving agent is the solvent. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. On the other hand, a suspension is a heterogeneous mixture where solid particles. Examples of colloids include milk, gelatin, and fog.
Suspension And Colloid Examples of colloids include milk, gelatin, and fog. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. The substance that is dissolved is the solute. On the other hand, a suspension is a heterogeneous mixture where solid particles. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Examples of colloids include milk, gelatin, and fog. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. The dissolving agent is the solvent. A solution is a homogeneous mixture of two or more components.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension And Colloid The dissolving agent is the solvent. The substance that is dissolved is the solute. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Because the dispersed particles of a colloid are not as. Suspension And Colloid.
From
Suspension And Colloid A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Because. Suspension And Colloid.
From
Suspension And Colloid The dissolving agent is the solvent. On the other hand, a suspension is a heterogeneous mixture where solid particles. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Examples of colloids include milk, gelatin, and fog. A solution is a homogeneous mixture of two or more components. Because the dispersed particles. Suspension And Colloid.
From www.youtube.com
Chemistry 9.4 Solutions, Colloids and Suspensions YouTube Suspension And Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. On the other hand, a suspension is a heterogeneous mixture where solid particles. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A solution is a homogeneous mixture. Suspension And Colloid.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension And Colloid The dissolving agent is the solvent. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Because the dispersed particles of a colloid are not. Suspension And Colloid.
From
Suspension And Colloid The dissolving agent is the solvent. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. On the other hand, a suspension is a heterogeneous mixture where solid particles. The substance that is dissolved. Suspension And Colloid.
From
Suspension And Colloid On the other hand, a suspension is a heterogeneous mixture where solid particles. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Examples of colloids include milk, gelatin, and fog. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A colloid is a mixture of tiny. Suspension And Colloid.
From
Suspension And Colloid On the other hand, a suspension is a heterogeneous mixture where solid particles. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Suspensions and colloids are two common types of mixtures whose properties. Suspension And Colloid.
From
Suspension And Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The substance that is dissolved is the solute. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Because the dispersed particles of a colloid are not as large. Suspension And Colloid.
From
Suspension And Colloid Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. On the other hand, a suspension is a heterogeneous mixture where solid particles. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Examples of. Suspension And Colloid.
From
Suspension And Colloid A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. The substance that is dissolved is the solute. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Suspensions and colloids are two common types of mixtures whose properties. Suspension And Colloid.
From
Suspension And Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The substance that is dissolved is. Suspension And Colloid.
From
Suspension And Colloid The substance that is dissolved is the solute. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke,. Suspension And Colloid.
From
Suspension And Colloid The substance that is dissolved is the solute. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. A solution is a homogeneous mixture of two or more components. Examples of colloids include milk, gelatin, and fog. On the other hand, a suspension is a heterogeneous mixture where solid particles. The dissolving agent is the solvent. A. Suspension And Colloid.
From
Suspension And Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Examples of colloids include milk, gelatin, and fog. Learn how. Suspension And Colloid.
From www.pinterest.com
solutionssuspensionsandcolloids9728.jpg (728×546) Science Suspension And Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Examples of colloids include milk, gelatin, and fog. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The substance that is dissolved is the solute. Learn the. Suspension And Colloid.
From
Suspension And Colloid A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle. Suspension And Colloid.
From
Suspension And Colloid Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one. Suspension And Colloid.
From
Suspension And Colloid Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A solution is a homogeneous. Suspension And Colloid.
From www.difference.wiki
Colloid vs. Suspension What’s the Difference? Suspension And Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Examples of colloids include milk,. Suspension And Colloid.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension And Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle. Suspension And Colloid.
From differencebtw.com
Suspension vs. Colloid Know the Difference Suspension And Colloid On the other hand, a suspension is a heterogeneous mixture where solid particles. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. The substance that is dissolved is the solute. A solution is a homogeneous mixture of two or more components. Solutions, suspensions, colloids, and other. Suspension And Colloid.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Suspension And Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. On the other hand, a. Suspension And Colloid.
From
Suspension And Colloid Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The dissolving agent is the solvent. The substance that is dissolved is the solute. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. A solution is a homogeneous mixture of two or more. Suspension And Colloid.
From
Suspension And Colloid Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical or chemical methods. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The substance that is dissolved is the solute. A colloid is a mixture of tiny particles dispersed in. Suspension And Colloid.
From www.youtube.com
Science 6 Q1 Week 2 Solution, Suspension, Colloid YouTube Suspension And Colloid A solution is a homogeneous mixture of two or more components. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. On the other hand, a suspension is a heterogeneous mixture where solid particles. A colloid is a mixture of tiny particles dispersed in another medium, such as. Suspension And Colloid.
From
Suspension And Colloid Examples of colloids include milk, gelatin, and fog. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. On the other hand, a suspension is a heterogeneous mixture where solid particles. Suspensions and colloids are two common types. Suspension And Colloid.
From
Suspension And Colloid The dissolving agent is the solvent. Examples of colloids include milk, gelatin, and fog. The substance that is dissolved is the solute. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. A colloid is a mixture of tiny particles dispersed in another medium, such as milk,. Suspension And Colloid.
From
Suspension And Colloid A solution is a homogeneous mixture of two or more components. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Learn the key differences between colloids and suspensions,. Suspension And Colloid.
From
Suspension And Colloid Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. On the other hand, a. Suspension And Colloid.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension And Colloid Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A solution is a homogeneous mixture of two or more components. Suspensions and colloids are two common types. Suspension And Colloid.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension And Colloid A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Learn how to distinguish a colloid from a solution or a suspension, and how to prepare a colloid using mechanical. Suspension And Colloid.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Suspension And Colloid Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. On the other hand, a suspension is a heterogeneous mixture where solid particles. Learn how to distinguish a. Suspension And Colloid.
From
Suspension And Colloid A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. Examples of colloids include milk, gelatin, and fog. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between. Suspension And Colloid.
From www.youtube.com
What is solution, Suspension and Colloid? Science Composition of Suspension And Colloid The dissolving agent is the solvent. A colloid is a mixture of tiny particles dispersed in another medium, such as milk, smoke, or gelatin. A solution is a homogeneous mixture of two or more components. Learn the key differences between colloids and suspensions, two types of heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that. Suspension And Colloid.