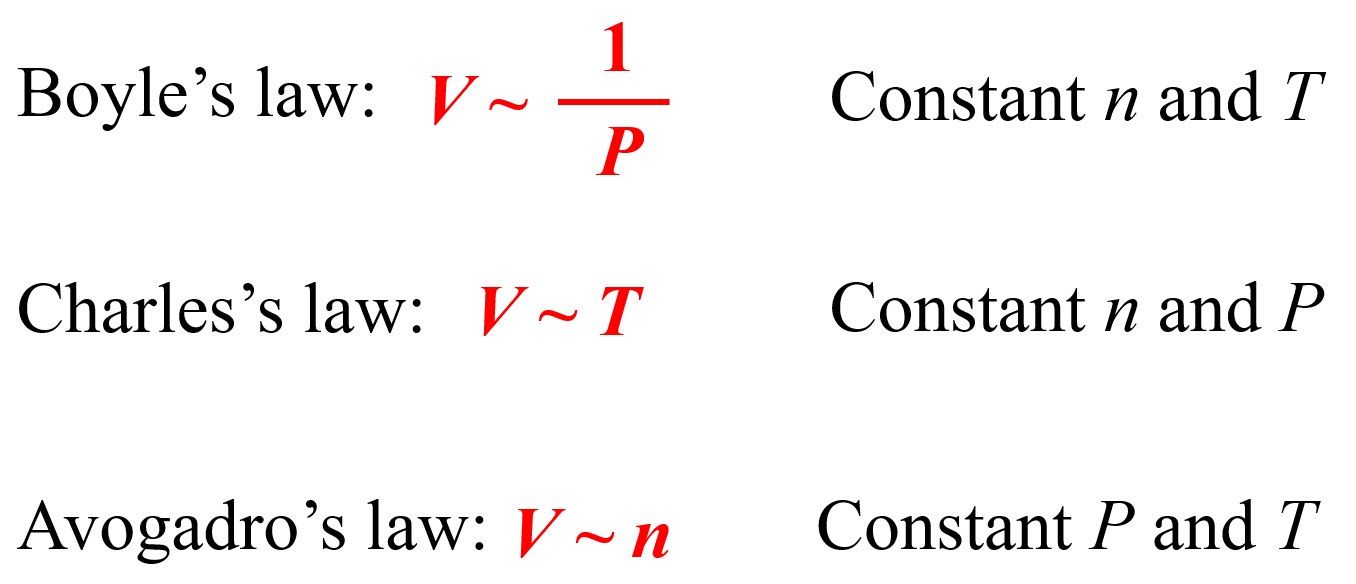Using The Ideal Gas Law And Your Experience In This Lab . The ideal gas law arises from several different gas laws. It relates the properties of pressure (p), volume (v), temperature (t), and. It is derived from the following laws: The ideal gas law is one of the most important relationships in science. When p is in atm, v is in liter, n is. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. Ideal gas law target students high school and introductory college physics and chemistry. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. Objectives to show that pv/t = nr. Pv = nrt where r is the ideal gas law constant.
from general.chemistrysteps.com
The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. Combining the individual gas laws, one gets the ideal gas law: It is derived from the following laws: When p is in atm, v is in liter, n is. Objectives to show that pv/t = nr. It relates the properties of pressure (p), volume (v), temperature (t), and. Ideal gas law target students high school and introductory college physics and chemistry. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The ideal gas law is one of the most important relationships in science.
The Ideal Gas Law Chemistry Steps
Using The Ideal Gas Law And Your Experience In This Lab It is derived from the following laws: Combining the individual gas laws, one gets the ideal gas law: It is derived from the following laws: Objectives to show that pv/t = nr. The ideal gas law arises from several different gas laws. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. It relates the properties of pressure (p), volume (v), temperature (t), and. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. When p is in atm, v is in liter, n is. The ideal gas law is one of the most important relationships in science. Ideal gas law target students high school and introductory college physics and chemistry. Pv = nrt where r is the ideal gas law constant. Boyle’s law describes the inverse relationship between pressure and volume, p ∝.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law arises from several different gas laws. Ideal gas law target students high school and introductory college physics and chemistry. Combining the individual gas laws, one gets the ideal gas law: It relates the properties of pressure (p), volume (v), temperature (t), and. The object of this experiment is to use the ideal gas model, from which. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studocu.com
Experiment Twenty Experiment 20 The Ideal Gas Laws 20 Introduction Using The Ideal Gas Law And Your Experience In This Lab The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. The ideal gas law arises from several different gas laws. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law utilises a variety of observable gas properties to create one universal equation. Using The Ideal Gas Law And Your Experience In This Lab.
From www.chegg.com
Solved Heat Ideal Gases and the First Law of Using The Ideal Gas Law And Your Experience In This Lab When p is in atm, v is in liter, n is. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. It relates the properties of pressure (p), volume (v), temperature (t), and. Ideal gas. Using The Ideal Gas Law And Your Experience In This Lab.
From plantecuador.com
Ideal Gas Law — Overview & Calculations Expii, ideal gas Using The Ideal Gas Law And Your Experience In This Lab Pv = nrt where r is the ideal gas law constant. When p is in atm, v is in liter, n is. It relates the properties of pressure (p), volume (v), temperature (t), and. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Ideal gas law target students. Using The Ideal Gas Law And Your Experience In This Lab.
From guide.openrif.org
How Do You Use Ideal Gas Law Guides Online Using The Ideal Gas Law And Your Experience In This Lab It is derived from the following laws: Combining the individual gas laws, one gets the ideal gas law: The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The object of this experiment is to use the. Using The Ideal Gas Law And Your Experience In This Lab.
From mmerevise.co.uk
The Ideal Gas Equation MME Using The Ideal Gas Law And Your Experience In This Lab Boyle’s law describes the inverse relationship between pressure and volume, p ∝. When p is in atm, v is in liter, n is. Pv = nrt where r is the ideal gas law constant. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. It relates the properties of. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
Master the Ideal Gas Law in Chemistry A StepbyStep Guide [1510 Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law arises from several different gas laws. The ideal gas law is one of the most important relationships in science. When p is in atm, v is in liter, n is. Ideal gas law target students high school and introductory college physics and chemistry. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. It. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Pv = nrt where r is the ideal gas law constant. It is derived from the following laws: Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The object of this experiment is to use the. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube Using The Ideal Gas Law And Your Experience In This Lab The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. It is derived from the following laws: Pv = nrt where r is the ideal gas law constant. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law arises from several different. Using The Ideal Gas Law And Your Experience In This Lab.
From chemistryguru.com.sg
Ideal Gas Law and Applications Using The Ideal Gas Law And Your Experience In This Lab When p is in atm, v is in liter, n is. Pv = nrt where r is the ideal gas law constant. Objectives to show that pv/t = nr. Ideal gas law target students high school and introductory college physics and chemistry. It relates the properties of pressure (p), volume (v), temperature (t), and. It is derived from the following. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
Calculate Specific Volume of Water Vapor Using Ideal Gas Law YouTube Using The Ideal Gas Law And Your Experience In This Lab It relates the properties of pressure (p), volume (v), temperature (t), and. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. Objectives to show that pv/t = nr. Pv = nrt where r is the ideal gas law constant. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law is one of. Using The Ideal Gas Law And Your Experience In This Lab.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Using The Ideal Gas Law And Your Experience In This Lab Ideal gas law target students high school and introductory college physics and chemistry. Combining the individual gas laws, one gets the ideal gas law: It is derived from the following laws: It relates the properties of pressure (p), volume (v), temperature (t), and. The ideal gas law is one of the most important relationships in science. When p is in. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studyxapp.com
chemistry ideal gas law constant introduction laboratory simulation a Using The Ideal Gas Law And Your Experience In This Lab Combining the individual gas laws, one gets the ideal gas law: Pv = nrt where r is the ideal gas law constant. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. It relates the properties of pressure (p), volume (v), temperature (t), and. The object of this experiment. Using The Ideal Gas Law And Your Experience In This Lab.
From studylib.net
ideal gas law lab Using The Ideal Gas Law And Your Experience In This Lab Boyle’s law describes the inverse relationship between pressure and volume, p ∝. Objectives to show that pv/t = nr. When p is in atm, v is in liter, n is. The ideal gas law is one of the most important relationships in science. Ideal gas law target students high school and introductory college physics and chemistry. The ideal gas law. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studocu.com
The ideal Gas laws using Charles' Laws and Boyle's Law Abstract For Using The Ideal Gas Law And Your Experience In This Lab It is derived from the following laws: The ideal gas law arises from several different gas laws. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law is one of the most important relationships in science. It relates the properties of pressure (p), volume (v), temperature (t), and. When p is in atm, v is. Using The Ideal Gas Law And Your Experience In This Lab.
From learncheme.com
idealgaslawconceptest1 LearnChemE Using The Ideal Gas Law And Your Experience In This Lab It relates the properties of pressure (p), volume (v), temperature (t), and. Objectives to show that pv/t = nr. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. The ideal gas law is one of the most important relationships in science. The ideal gas law arises from several. Using The Ideal Gas Law And Your Experience In This Lab.
From www.yumpu.com
Lab 12 The Ideal Gas Law Using The Ideal Gas Law And Your Experience In This Lab Ideal gas law target students high school and introductory college physics and chemistry. Objectives to show that pv/t = nr. It is derived from the following laws: It relates the properties of pressure (p), volume (v), temperature (t), and. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the. Using The Ideal Gas Law And Your Experience In This Lab.
From www.slideserve.com
PPT IDEAL GAS LAW PowerPoint Presentation, free download ID4200155 Using The Ideal Gas Law And Your Experience In This Lab Objectives to show that pv/t = nr. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. Pv = nrt where r is the ideal gas law constant. It is derived from the following laws: When p is in atm, v is in liter, n is. Boyle’s. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With Using The Ideal Gas Law And Your Experience In This Lab Boyle’s law describes the inverse relationship between pressure and volume, p ∝. It is derived from the following laws: The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Ideal gas law target students high school and introductory college physics and chemistry. It relates the properties of pressure (p),. Using The Ideal Gas Law And Your Experience In This Lab.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Using The Ideal Gas Law And Your Experience In This Lab The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. It relates the properties of pressure (p), volume (v), temperature (t), and. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. When p is in atm, v is in liter, n is. Objectives to. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studypool.com
SOLUTION Ideal Gas Law Notes Studypool Using The Ideal Gas Law And Your Experience In This Lab When p is in atm, v is in liter, n is. Ideal gas law target students high school and introductory college physics and chemistry. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. It. Using The Ideal Gas Law And Your Experience In This Lab.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Using The Ideal Gas Law And Your Experience In This Lab Pv = nrt where r is the ideal gas law constant. Objectives to show that pv/t = nr. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. The ideal gas law arises from several different gas laws. It relates the properties of pressure (p), volume (v),. Using The Ideal Gas Law And Your Experience In This Lab.
From www.pearson.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT Pearson+ Channels Using The Ideal Gas Law And Your Experience In This Lab The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. When p is in atm, v is in liter, n is. It relates the properties of pressure (p), volume (v), temperature (t), and. Objectives to show that pv/t = nr. The ideal gas law utilises a variety. Using The Ideal Gas Law And Your Experience In This Lab.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe Using The Ideal Gas Law And Your Experience In This Lab The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. Combining the individual gas laws, one gets the ideal gas law: Ideal gas law target students high school and introductory college physics and chemistry. When p is in atm, v is in liter, n is. Pv =. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studypool.com
SOLUTION Ideal Gas Lab Report Studypool Using The Ideal Gas Law And Your Experience In This Lab Ideal gas law target students high school and introductory college physics and chemistry. The ideal gas law is one of the most important relationships in science. Combining the individual gas laws, one gets the ideal gas law: Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The ideal gas law arises from several different gas laws. Objectives. Using The Ideal Gas Law And Your Experience In This Lab.
From www.chegg.com
Solved Part I Using the Ideal Gas Law Experiment 1 Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law arises from several different gas laws. When p is in atm, v is in liter, n is. It is derived from the following laws: Combining the individual gas laws, one gets the ideal gas law: The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the. Using The Ideal Gas Law And Your Experience In This Lab.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Using The Ideal Gas Law And Your Experience In This Lab Pv = nrt where r is the ideal gas law constant. The ideal gas law arises from several different gas laws. It is derived from the following laws: The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Combining the individual gas laws, one gets the ideal gas law:. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube Using The Ideal Gas Law And Your Experience In This Lab When p is in atm, v is in liter, n is. The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. It is derived from the following laws: Combining the individual gas laws, one gets the ideal gas law: It relates the properties of pressure (p), volume. Using The Ideal Gas Law And Your Experience In This Lab.
From studylib.net
Ideal Gas Law Using The Ideal Gas Law And Your Experience In This Lab Objectives to show that pv/t = nr. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. The ideal gas law arises from several different gas laws. Combining the individual gas laws, one gets the ideal gas. Using The Ideal Gas Law And Your Experience In This Lab.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Using The Ideal Gas Law And Your Experience In This Lab Pv = nrt where r is the ideal gas law constant. The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Ideal gas law target students high school and introductory college physics and chemistry. Combining the individual gas laws, one gets the ideal gas law: The ideal gas law. Using The Ideal Gas Law And Your Experience In This Lab.
From sciencenotes.org
Ideal Gas Law Formula and Examples Using The Ideal Gas Law And Your Experience In This Lab Ideal gas law target students high school and introductory college physics and chemistry. It is derived from the following laws: Objectives to show that pv/t = nr. Pv = nrt where r is the ideal gas law constant. Combining the individual gas laws, one gets the ideal gas law: It relates the properties of pressure (p), volume (v), temperature (t),. Using The Ideal Gas Law And Your Experience In This Lab.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. When p is in atm, v is in liter, n is. Pv = nrt where r is the ideal gas law constant. It relates the properties of pressure (p), volume (v), temperature (t), and. Ideal gas law target students. Using The Ideal Gas Law And Your Experience In This Lab.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube Using The Ideal Gas Law And Your Experience In This Lab It is derived from the following laws: Combining the individual gas laws, one gets the ideal gas law: The object of this experiment is to use the ideal gas model, from which a simple relationship between the pressure, the volume, the. It relates the properties of pressure (p), volume (v), temperature (t), and. When p is in atm, v is. Using The Ideal Gas Law And Your Experience In This Lab.
From www.vectorstock.com
Ideal gas law boyles law pressure volume Vector Image Using The Ideal Gas Law And Your Experience In This Lab The ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. The ideal gas law is one of the most important relationships in science. The ideal gas law arises from several different gas laws. Objectives to show that pv/t = nr. Pv = nrt where r is the ideal gas. Using The Ideal Gas Law And Your Experience In This Lab.
From www.studocu.com
Ideal gas law lab phet.colorado/ Open the Phet simulation Using The Ideal Gas Law And Your Experience In This Lab Pv = nrt where r is the ideal gas law constant. It relates the properties of pressure (p), volume (v), temperature (t), and. When p is in atm, v is in liter, n is. Objectives to show that pv/t = nr. The ideal gas law is one of the most important relationships in science. The ideal gas law utilises a. Using The Ideal Gas Law And Your Experience In This Lab.