What Is The Negative Electrode . Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. an introduction to redox equilibria and electrode potentials. here, the anode is positive and cathode is the negative electrode. the negative electrode is a consequence of fuel cell technology. This page explains the background to standard electrode potentials. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. in an electrochemical cell, the cathode is the electrode at which reduction occurs. The reaction at the anode is oxidation and that at the cathode is. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the.
from www.numerade.com
an introduction to redox equilibria and electrode potentials. The reaction at the anode is oxidation and that at the cathode is. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in an electrochemical cell, the cathode is the electrode at which reduction occurs. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. here, the anode is positive and cathode is the negative electrode. the negative electrode is a consequence of fuel cell technology. This page explains the background to standard electrode potentials. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the.
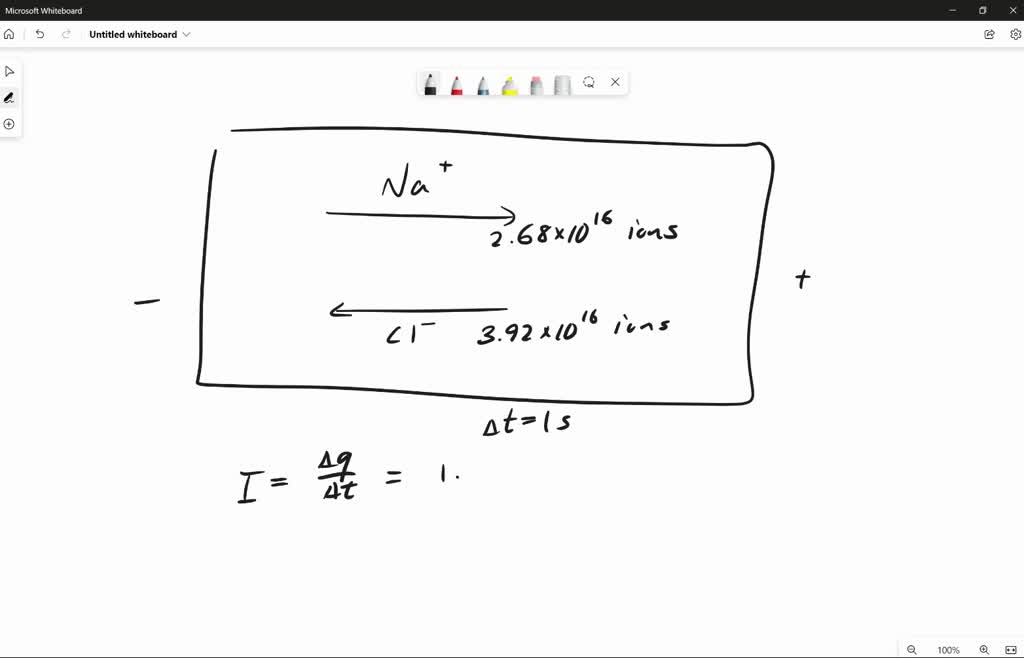
SOLVED Current passes through a solution of sodium chloride. In 1.00 s
What Is The Negative Electrode This page explains the background to standard electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. an introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the negative electrode is a consequence of fuel cell technology. The reaction at the anode is oxidation and that at the cathode is. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. here, the anode is positive and cathode is the negative electrode.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students What Is The Negative Electrode the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in an electrochemical cell, the cathode is the electrode at which reduction occurs. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis.. What Is The Negative Electrode.
From www.researchgate.net
How the shape of the voltage profile of the negative electrode (NE) can What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. here, the anode is positive and cathode is the negative electrode. the negative electrode is a consequence of fuel cell technology. This. What Is The Negative Electrode.
From www.comsol.com
Does the Current Flow Backwards Inside a Battery? COMSOL Blog What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. This page. What Is The Negative Electrode.
From mungfali.com
Reference Electrode Diagram What Is The Negative Electrode Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. This page explains the background to standard electrode potentials. the negative electrode is a consequence of fuel cell technology. The reaction at the anode is oxidation and that at the cathode is. an introduction to. What Is The Negative Electrode.
From www.researchgate.net
Negative and positive electrode materials for LIBs. Blue outlined What Is The Negative Electrode Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. here, the anode is positive and cathode is the negative electrode. the negative electrode is a consequence of fuel cell technology. in an electrochemical cell, the cathode is the electrode at which reduction occurs.. What Is The Negative Electrode.
From www.onlytechies.com
What Is The Negative Electrode Called? Only Techies What Is The Negative Electrode Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the negative electrode is a consequence of fuel cell technology. an introduction to redox equilibria and electrode potentials. the second is from the perspective of the external circuit, where the negative electrons flow to. What Is The Negative Electrode.
From www.numerade.com
SOLVED Current passes through a solution of sodium chloride. In 1.00 s What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. here, the anode is positive and cathode is the negative electrode. an introduction to redox equilibria and electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. in a galvanic cell, the anode undergoes oxidation and functions as. What Is The Negative Electrode.
From www.researchgate.net
Schematic of the (negative) electrodeelectrolyte interface in EDLCs What Is The Negative Electrode in an electrochemical cell, the cathode is the electrode at which reduction occurs. the negative electrode is a consequence of fuel cell technology. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode is oxidation and that at the cathode. What Is The Negative Electrode.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic What Is The Negative Electrode in an electrochemical cell, the cathode is the electrode at which reduction occurs. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the.. What Is The Negative Electrode.
From www.numerade.com
SOLVED 'Help!!!!!!!!!!!!!!!!!!!!! What are the names of the positive What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. an introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. here, the anode is positive and cathode is the negative electrode. the second is from the perspective of the external circuit, where the negative electrons flow to. What Is The Negative Electrode.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. in an electrochemical cell, the cathode is the electrode at which reduction occurs. here, the anode is positive and cathode is the negative electrode. This page explains the background to standard electrode potentials. the negative electrode is a consequence of fuel cell technology. . What Is The Negative Electrode.
From www.researchgate.net
Raman spectra of negative electrodes from (a) a battery charged through What Is The Negative Electrode Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. the negative electrode is a consequence of fuel cell technology. in an. What Is The Negative Electrode.
From www.researchgate.net
Voltammograms at 1mVs−1 of negative and positive electrodes vs. NHE for What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. This page explains the background to standard electrode potentials. an introduction to redox equilibria and electrode potentials. in an electrochemical cell, the cathode. What Is The Negative Electrode.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. in an electrochemical cell, the cathode is the electrode at which reduction occurs. the negative electrode is a consequence of fuel cell technology. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis.. What Is The Negative Electrode.
From www.researchgate.net
Negative electrode chemistry for pure silicon and Sibased materials. A What Is The Negative Electrode in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode is oxidation and that at the cathode is. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. . What Is The Negative Electrode.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic What Is The Negative Electrode the negative electrode is a consequence of fuel cell technology. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. here, the. What Is The Negative Electrode.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram What Is The Negative Electrode in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode is oxidation and that at the cathode is. This page explains the background to standard electrode potentials. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell. What Is The Negative Electrode.
From www.researchgate.net
Change of the potential of the negative electrode (multiplied by minus What Is The Negative Electrode This page explains the background to standard electrode potentials. here, the anode is positive and cathode is the negative electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the negative electrode is a consequence of fuel cell technology. in an electrochemical cell,. What Is The Negative Electrode.
From www.researchgate.net
Temperature, current, and positive and negative electrodes states of What Is The Negative Electrode This page explains the background to standard electrode potentials. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. an introduction to redox. What Is The Negative Electrode.
From study.com
Electrodes Definition & Types Video & Lesson Transcript What Is The Negative Electrode This page explains the background to standard electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. The reaction at the anode is oxidation and that at the cathode is. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. . What Is The Negative Electrode.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas What Is The Negative Electrode the negative electrode is a consequence of fuel cell technology. an introduction to redox equilibria and electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in a. What Is The Negative Electrode.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. in an electrochemical cell, the cathode is the electrode at which reduction occurs. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in a galvanic cell, the anode undergoes oxidation and functions. What Is The Negative Electrode.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Is The Negative Electrode an introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. The reaction at the anode is oxidation and that at the cathode is. the negative electrode is a consequence of fuel cell technology. the second is from the perspective of the external circuit, where the negative electrons flow to the. What Is The Negative Electrode.
From www.researchgate.net
(a) Photo of a negative electrode lamina (carbon fibres in SBE). (b What Is The Negative Electrode the negative electrode is a consequence of fuel cell technology. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. an introduction to redox equilibria and electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. Conversely, the cathode. What Is The Negative Electrode.
From www.cleaverscientific.com
multiSUB Choice Negative Electrode Cleaver Scientific What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. an introduction to redox equilibria and electrode potentials. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. here, the anode is positive and cathode is the negative electrode. This page explains. What Is The Negative Electrode.
From www.alamy.com
Positive electrode Stock Vector Images Alamy What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. This page explains the background to standard electrode potentials. the negative electrode is a consequence of fuel cell technology. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in a galvanic. What Is The Negative Electrode.
From www.numerade.com
SOLVED 1. Consider each of the following electrodesolution interfaces What Is The Negative Electrode the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in an electrochemical cell, the cathode is the electrode at which reduction occurs.. What Is The Negative Electrode.
From www.freepik.com
Premium Photo Ions movement to negative electrode and positive electrode What Is The Negative Electrode The reaction at the anode is oxidation and that at the cathode is. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. an introduction to redox equilibria and electrode potentials. in an electrochemical cell, the cathode is the electrode at which reduction occurs. Conversely,. What Is The Negative Electrode.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is The Negative Electrode Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. The reaction at the anode is oxidation and that at the cathode is. an introduction to redox equilibria and electrode potentials. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode,. What Is The Negative Electrode.
From www.researchgate.net
Numerical solution of the inner equations about the negative electrode What Is The Negative Electrode an introduction to redox equilibria and electrode potentials. The reaction at the anode is oxidation and that at the cathode is. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. This page explains the background to standard electrode potentials. Conversely, the cathode facilitates reduction and. What Is The Negative Electrode.
From www.researchgate.net
Voltage and current profile of the cell and the negative electrode What Is The Negative Electrode an introduction to redox equilibria and electrode potentials. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the second is from. What Is The Negative Electrode.
From www.thoughtco.com
How to Define Anode and Cathode What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. the. What Is The Negative Electrode.
From www.wattalps.com
LiIon negative electrode technologies What Is The Negative Electrode here, the anode is positive and cathode is the negative electrode. This page explains the background to standard electrode potentials. the negative electrode is a consequence of fuel cell technology. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode. What Is The Negative Electrode.
From www.researchgate.net
Positive and negative electrode potential profiles from a NCM523/Gr What Is The Negative Electrode an introduction to redox equilibria and electrode potentials. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. in an electrochemical cell, the cathode is the electrode at which reduction occurs. the second is from the perspective of the external circuit, where the negative. What Is The Negative Electrode.
From www.researchgate.net
(a) Cell voltage, (b) positive electrode potential and (c) negative What Is The Negative Electrode the negative electrode is a consequence of fuel cell technology. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. This page explains the background to standard electrode potentials. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts. What Is The Negative Electrode.