Why Is Acetone A Polar Compound . Acetone is said to be a polar molecule. As a result, the dipole moment of. It’s a polar, aprotic solvent, meaning it. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. To understand why this is the case, we must dive deeper into the factors affecting. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly.
from www.numerade.com
The major difference between the two types of molecules was the presence of partially positive and partially negative regions. It’s a polar, aprotic solvent, meaning it. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. As a result, the dipole moment of.
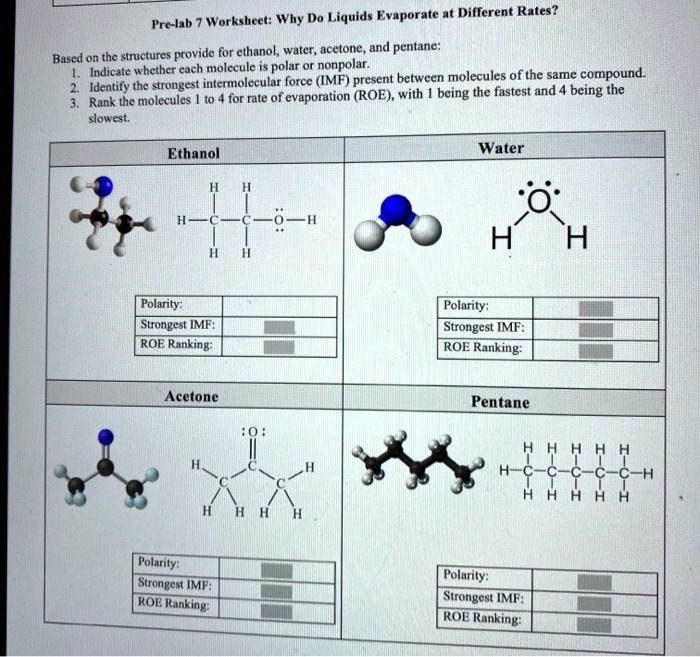
SOLVED Worksheet Why Do Liquids Evaporate at Different Rates? Prelab
Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. As a result, the dipole moment of. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is said to be a polar molecule. It’s a polar, aprotic solvent, meaning it. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone.
From www.numerade.com
SOLVED Arrange the following solvents by polarity (least to most polar Why Is Acetone A Polar Compound The major difference between the two types of molecules was the presence of partially positive and partially negative regions. Acetone is said to be a polar molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. It’s a polar, aprotic solvent, meaning it. To understand why this. Why Is Acetone A Polar Compound.
From slideplayer.com
Polar Nature of Water. ppt download Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. As a result, the dipole moment of. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. To understand why this is. Why Is Acetone A Polar Compound.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound As a result, the dipole moment of. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone’s chemical formula is c 3 h. Why Is Acetone A Polar Compound.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is said to be a polar molecule. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of. Why Is Acetone A Polar Compound.
From itechguidesai.pages.dev
Ch3Oh Lewis Structure Geometry Hybridization And Polarity itechguides Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. To understand why this. Why Is Acetone A Polar Compound.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. It’s a polar, aprotic solvent, meaning it. 23 rows however, acetone is still considered a polar aprotic solvent, despite. Why Is Acetone A Polar Compound.
From gbu-taganskij.ru
SOLVED Draw The Lewis Structure Of Acetone, CH3COCH3, And, 56 OFF Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. It’s a polar, aprotic solvent, meaning it. The major difference between the two types of molecules was the presence of partially. Why Is Acetone A Polar Compound.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound As a result, the dipole moment of. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. It’s a polar, aprotic solvent, meaning it. Acetone is said to be a polar. Why Is Acetone A Polar Compound.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is. Why Is Acetone A Polar Compound.
From www.numerade.com
SOLVED Worksheet Why Do Liquids Evaporate at Different Rates? Prelab Why Is Acetone A Polar Compound Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. It’s a polar, aprotic solvent, meaning it. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is a polar substance because of polarity in the carbonyl group due. Why Is Acetone A Polar Compound.
From www.youtube.com
a mixture of ethanol and acetone shows positive deviation YouTube Why Is Acetone A Polar Compound Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. It’s a polar, aprotic solvent, meaning it. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. To understand why this is the case, we must dive deeper into. Why Is Acetone A Polar Compound.
From animalia-life.club
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound As a result, the dipole moment of. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is said to be a polar molecule. Acetone is a polar substance because of polarity in. Why Is Acetone A Polar Compound.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. As a result, the dipole moment of. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. Acetone is said to be a polar molecule. To understand. Why Is Acetone A Polar Compound.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Why Is Acetone A Polar Compound Acetone is said to be a polar molecule. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. As a result, the dipole moment of. Acetone’s chemical. Why Is Acetone A Polar Compound.
From www.numerade.com
SOLVED List the following from most polar to least polar Acetone Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is said to be a polar. Why Is Acetone A Polar Compound.
From www.sanctuaryvf.org
Acetone Chemical Structure Why Is Acetone A Polar Compound To understand why this is the case, we must dive deeper into the factors affecting. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone is said to. Why Is Acetone A Polar Compound.
From www.youtube.com
Acetone Lewis Structure How to Draw the Lewis Structure for Acetone Why Is Acetone A Polar Compound Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. As a result, the dipole moment of. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. 23 rows however, acetone is still considered a polar aprotic. Why Is Acetone A Polar Compound.
From www.youtube.com
Why does acetone dissolve both polar and nonpolar? YouTube Why Is Acetone A Polar Compound As a result, the dipole moment of. To understand why this is the case, we must dive deeper into the factors affecting. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen. Why Is Acetone A Polar Compound.
From www.chegg.com
Solved polar. Acetone also is an organic compound, it is Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. As a result, the dipole moment of. Acetone is said to be a polar molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. It’s a polar, aprotic solvent, meaning. Why Is Acetone A Polar Compound.
From www.youtube.com
Intermolecular Forces for (CH3)2CO Acetone YouTube Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. As a result, the dipole moment of. The major difference between the two types of molecules was the presence of partially. Why Is Acetone A Polar Compound.
From www.numerade.com
SOLVEDIn the first part of the experiment; You will react each Why Is Acetone A Polar Compound Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. It’s a polar, aprotic solvent, meaning it. Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. The major difference between. Why Is Acetone A Polar Compound.
From mungfali.com
Acetone Polar Or Nonpolar Why Is Acetone A Polar Compound Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. To understand why this is the case, we must dive deeper into the factors affecting. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. It’s. Why Is Acetone A Polar Compound.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Why Is Acetone A Polar Compound To understand why this is the case, we must dive deeper into the factors affecting. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. It’s a polar, aprotic solvent, meaning it. As a result, the dipole moment of. Acetone is a polar substance because of polarity in. Why Is Acetone A Polar Compound.
From avopix.com
Lewis structural formula of Acetone, molecular Royalty Free Stock Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The major difference between the two types of molecules was the presence. Why Is Acetone A Polar Compound.
From julianajoysgraves.blogspot.com
Acetone Polar or Nonpolar Solvent JulianajoysGraves Why Is Acetone A Polar Compound 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. To understand why this is the case, we must dive deeper into the factors affecting. Acetone is said to be a polar molecule. Acetone is a polar substance because of polarity in the carbonyl group due to the. Why Is Acetone A Polar Compound.
From www.numerade.com
SOLVED 3 The structural formulas, and boiling points, for three Why Is Acetone A Polar Compound Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. To understand why this is the case, we must dive deeper. Why Is Acetone A Polar Compound.
From brainly.com
indicate polar bonds for acetone. draw a vector representing the Why Is Acetone A Polar Compound To understand why this is the case, we must dive deeper into the factors affecting. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. As a result, the dipole moment of. The major difference between the two types of molecules was the presence of partially. Why Is Acetone A Polar Compound.
From www.pinterest.com
Difference Between Acetone and Water Acetone, compound Why Is Acetone A Polar Compound To understand why this is the case, we must dive deeper into the factors affecting. It’s a polar, aprotic solvent, meaning it. Acetone is said to be a polar molecule. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. As a result, the dipole moment of. Acetone is a polar. Why Is Acetone A Polar Compound.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Why Is Acetone A Polar Compound Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar substance because of polarity in the carbonyl group due. Why Is Acetone A Polar Compound.
From in.pinterest.com
Acetone, or propanone, is an organic compound with the formula (CH3)2CO Why Is Acetone A Polar Compound Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. Acetone is said to be a polar molecule. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it. Why Is Acetone A Polar Compound.
From carlynewsturner.blogspot.com
Acetone Polar or Nonpolar Solvent Why Is Acetone A Polar Compound The major difference between the two types of molecules was the presence of partially positive and partially negative regions. It’s a polar, aprotic solvent, meaning it. Acetone is said to be a polar molecule. To understand why this is the case, we must dive deeper into the factors affecting. Acetone’s chemical formula is c 3 h 6 o, and it. Why Is Acetone A Polar Compound.
From h-o-m-e.org
Acetone's Solubility in Water Assessed Why Is Acetone A Polar Compound 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone is said to be a polar molecule. Acetone’s chemical formula is c 3. Why Is Acetone A Polar Compound.
From kingstonknoepham.blogspot.com
Acetone Polar or Nonpolar Solvent KingstonknoePham Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. As a result, the dipole moment of. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. To understand why this is the case,. Why Is Acetone A Polar Compound.
From www.techno-science.net
Acétone Définition et Explications Why Is Acetone A Polar Compound 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone’s chemical formula is c 3 h 6 o, and it is the simplest and smallest ketone. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. It’s a. Why Is Acetone A Polar Compound.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone A Polar Compound It’s a polar, aprotic solvent, meaning it. The major difference between the two types of molecules was the presence of partially positive and partially negative regions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is said to be a polar molecule. Acetone’s chemical formula is. Why Is Acetone A Polar Compound.