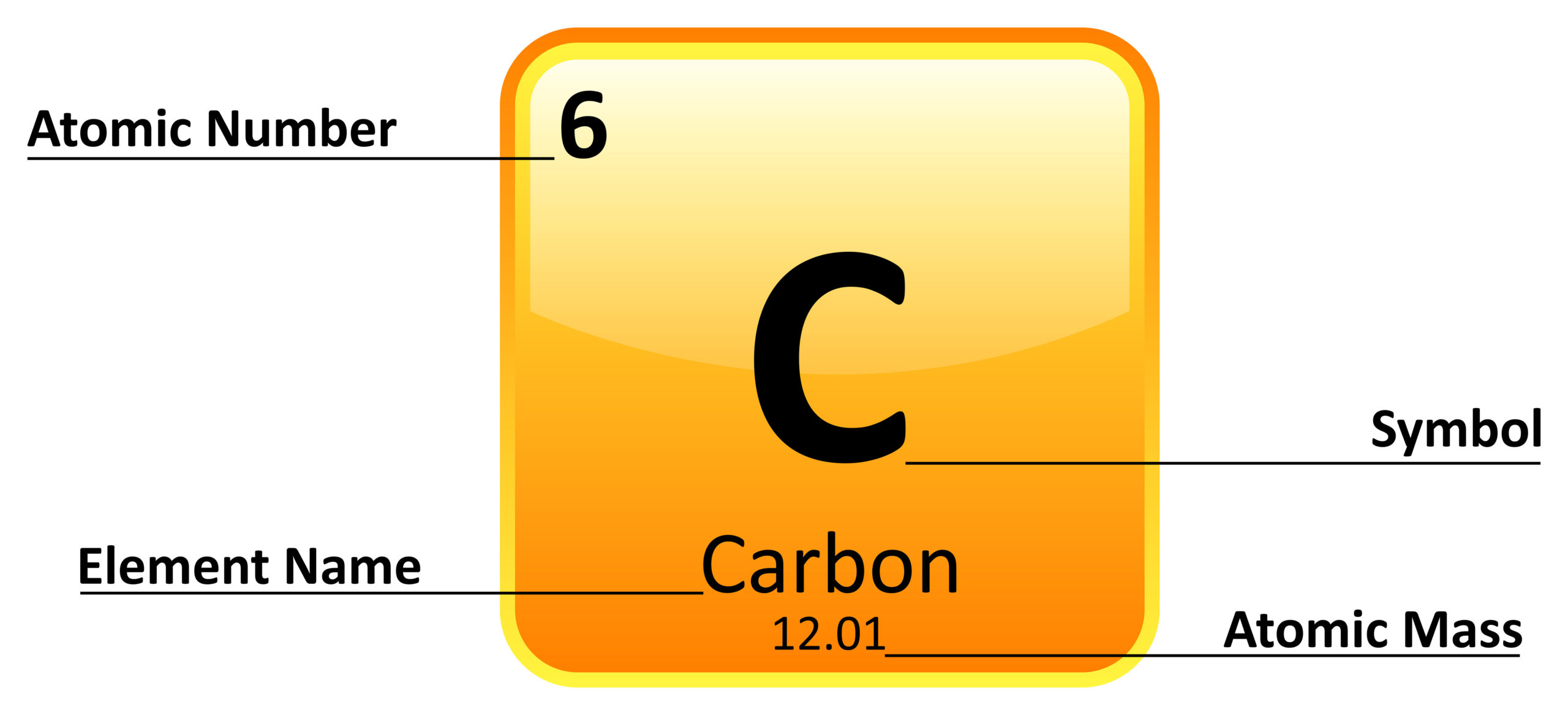
Element X Atomic Mass . When one or more electrons are. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. View rotating bohr models for all 118 elements. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Access detailed info on all elements: This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. It is simple to learn how to calculate the atomic mass of. Get a free hd image of the periodic. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. The atomic weight is the average mass in atomic mass units of an element’s atoms. Elements usually have more than one isotope; Atomic mass, electron configurations, charges, and more. An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number.
from www.animalia-life.club
Access detailed info on all elements: The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. View rotating bohr models for all 118 elements. The atomic weight is the average mass in atomic mass units of an element’s atoms. When one or more electrons are. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Get a free hd image of the periodic. Elements usually have more than one isotope; It is simple to learn how to calculate the atomic mass of. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u.
Periodic Table Of Elements With Names And Symbols And Atomic Mass And Atomic Number For Kids
Element X Atomic Mass When one or more electrons are. The atomic weight is the average mass in atomic mass units of an element’s atoms. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. When one or more electrons are. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Get a free hd image of the periodic. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. View rotating bohr models for all 118 elements. An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. Atomic mass, electron configurations, charges, and more. It is simple to learn how to calculate the atomic mass of. Elements usually have more than one isotope; Access detailed info on all elements: The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
From www.animalia-life.club
Periodic Table Of Elements With Full Names And Symbols And Atomic Mass And Atomic Number Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Get a free hd image of the periodic. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Elements usually have more than one isotope; Access detailed info. Element X Atomic Mass.
From pubchemblog.ncbi.nlm.nih.gov
Atomic mass changes in PubChem PubChem Blog Element X Atomic Mass The atomic weight is the average mass in atomic mass units of an element’s atoms. View rotating bohr models for all 118 elements. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. An isotope of any element can be uniquely represented as a. Element X Atomic Mass.
From periodictable.me
Way to Find Atomic Mass of Elements with Examples Element X Atomic Mass The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Atomic mass, electron configurations, charges, and more. When one or more electrons are. The atomic weight is the. Element X Atomic Mass.
From revisionscience.com
Atomic Number and Mass Number ALevel Chemistry Element X Atomic Mass It is simple to learn how to calculate the atomic mass of. Access detailed info on all elements: The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Elements usually have more than one isotope; Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. When one. Element X Atomic Mass.
From mungfali.com
Periodic Table Of Elements With Atomic Mass Numbers Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. It is simple to learn how to calculate the atomic mass of. Elements usually have more than one isotope; This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass. Element X Atomic Mass.
From sciencenotes.org
Periodic Table with Atomic Mass Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. View rotating bohr models for all 118 elements. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. The atomic weight is the average mass in atomic mass. Element X Atomic Mass.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol Element X Atomic Mass Elements usually have more than one isotope; When one or more electrons are. The atomic weight is the average mass in atomic mass units of an element’s atoms. It is simple to learn how to calculate the atomic mass of. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. View rotating bohr models for all 118 elements. The atomic mass of an element is. Element X Atomic Mass.
From periodictable.me
Modern Periodic Table of Elements with Names and Symbols Element X Atomic Mass The atomic weight is the average mass in atomic mass units of an element’s atoms. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. Atomic mass, electron configurations, charges, and more. The atomic mass of an element is. Element X Atomic Mass.
From nursehub.com
Atomic Number and Atomic Mass NurseHub Element X Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Access detailed info on all elements: When one or more electrons are. Atomic mass, electron configurations, charges, and more. The atomic mass of an element is the weighted average of the masses of the. Element X Atomic Mass.
From www.doubtnut.com
An element 'x' (Atomic mass = 40 g mol^(1)) having f.c.c. structure has unit cell edge length Element X Atomic Mass The atomic weight is the average mass in atomic mass units of an element’s atoms. Elements usually have more than one isotope; The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Get a free hd image of the periodic. It is simple to learn how to calculate the atomic mass of.. Element X Atomic Mass.
From www.animalia-life.club
Periodic Table Of Elements With Names And Symbols And Atomic Mass And Atomic Number For Kids Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Access detailed info on all elements: The atomic weight is the average mass in atomic mass units of an element’s atoms. When one or more electrons are. The atomic mass of an element is the weighted average of. Element X Atomic Mass.
From chem.libretexts.org
4.5 Chemical Symbols and the Atomic Number Chemistry LibreTexts Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. View rotating bohr models for all 118 elements. Elements usually have more than one isotope; When one or more electrons are. An isotope of any element can be uniquely represented as a z x, where x is the. Element X Atomic Mass.
From www.thoughtco.com
Periodic Table of the Elements Accepted Atomic Masses Element X Atomic Mass View rotating bohr models for all 118 elements. Get a free hd image of the periodic. It is simple to learn how to calculate the atomic mass of. An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic. Element X Atomic Mass.
From www.tpsearchtool.com
Periodic Table Of Elements With Names And Symbols And Atomic Mass And Images Element X Atomic Mass An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. 0.7577(34.969u). Element X Atomic Mass.
From www.animalia-life.club
Modern Periodic Table With Atomic Mass And Atomic Number Element X Atomic Mass View rotating bohr models for all 118 elements. The atomic weight is the average mass in atomic mass units of an element’s atoms. Get a free hd image of the periodic. When one or more electrons are. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the. Element X Atomic Mass.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Elements and Chemistry Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Access detailed info on all elements: Atomic mass, electron configurations, charges, and more. The atomic weight is the average mass in atomic mass units of an element’s atoms. The atomic mass of an element is a weighted average. Element X Atomic Mass.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Element X Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Get a free hd image of the periodic. Access detailed info on all elements: View rotating bohr models for all 118 elements. The atomic weight is the average mass in atomic mass units of. Element X Atomic Mass.
From www.thoughtco.com
A List of All the Elements of the Periodic Table Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. When one or more electrons are. Get a free hd image of the periodic. It is simple to learn how. Element X Atomic Mass.
From sciencenotes.org
Periodic Table with Atomic Mass Element X Atomic Mass The atomic weight is the average mass in atomic mass units of an element’s atoms. It is simple to learn how to calculate the atomic mass of. Get a free hd image of the periodic. Atomic mass, electron configurations, charges, and more. An isotope of any element can be uniquely represented as a z x, where x is the atomic. Element X Atomic Mass.
From mungfali.com
Periodic Table With Names And Atomic Mass Element X Atomic Mass Get a free hd image of the periodic. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. It is simple to learn how to calculate the atomic. Element X Atomic Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Element X Atomic Mass View rotating bohr models for all 118 elements. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. It is simple to learn how to calculate the atomic mass of. The atomic mass of an element is the weighted average of the atomic masses. Element X Atomic Mass.
From www.chemicool.com
Periodic Table of Elements with Relative Atomic Masses Element X Atomic Mass An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. Atomic mass, electron configurations, charges, and more. Elements usually have more than one isotope; The atomic mass of an element is the weighted average of the masses. Element X Atomic Mass.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Element X Atomic Mass An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. It is simple to learn how to calculate the atomic mass of. The atomic mass of an element is a weighted average of all the element's isotopes. Element X Atomic Mass.
From tinnongtuyensinh.com
What Are The 1 To 30 Elements Exploring The First Thirty Elements Of The Periodic Table Element X Atomic Mass The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. The atomic weight is the average mass in atomic. Element X Atomic Mass.
From courses.lumenlearning.com
The Periodic Table Chemistry for Majors Element X Atomic Mass It is simple to learn how to calculate the atomic mass of. Access detailed info on all elements: Elements usually have more than one isotope; The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. An isotope of any element can be uniquely represented as a z. Element X Atomic Mass.
From ar.inspiredpencil.com
Periodic Table Of Elements With Atomic Mass Element X Atomic Mass It is simple to learn how to calculate the atomic mass of. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Access detailed info on all elements: The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. This atomic. Element X Atomic Mass.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts Britannica Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Get a free hd image of the periodic. It is simple to learn how to calculate the atomic mass of. When one or more electrons are. View rotating bohr models for all 118 elements. Atomic mass, electron configurations,. Element X Atomic Mass.
From atomic-zz.blogspot.com
24 TUTORIAL PERIODIC TABLE CALCULATE ATOMIC MASS WITH PDF AND VIDEO Atomic Element X Atomic Mass Atomic mass, electron configurations, charges, and more. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. When one or more electrons are. Elements usually have more than one isotope; It is simple to learn how to calculate the. Element X Atomic Mass.
From courses.lumenlearning.com
The Periodic Table of Elements Biology for Majors I Element X Atomic Mass Atomic mass, electron configurations, charges, and more. It is simple to learn how to calculate the atomic mass of. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Elements usually have more than one isotope; The atomic weight is the average mass in atomic mass units of an element’s. Element X Atomic Mass.
From classnotes.ng
Atomic Number, Mass Number, Isotopes And Calculations ClassNotes.ng Element X Atomic Mass Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. Access detailed info on all elements: An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. The atomic weight is the average. Element X Atomic Mass.
From topperssocialscience.blogspot.com
Atomic Mass of Elements Atomic Mass and Valency of First 30 Elements Element X Atomic Mass An isotope of any element can be uniquely represented as a z x, where x is the atomic symbol of the element, a is the mass number and z is the atomic number. The atomic weight is the average mass in atomic mass units of an element’s atoms. It is simple to learn how to calculate the atomic mass of.. Element X Atomic Mass.
From alquilercastilloshinchables.info
7 Images Periodic Table With Names And Atomic Mass Number Valency And Review Alqu Blog Element X Atomic Mass The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. View rotating bohr models for all 118 elements. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. Elements usually have more than one isotope; This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic. Element X Atomic Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Element X Atomic Mass View rotating bohr models for all 118 elements. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. When one or more electrons are. 0.7577(34.969u) + 0.2423(36.966u) = 35.453u. Access detailed info on all elements: The atomic mass of an element is the weighted average of the masses. Element X Atomic Mass.
From www.youtube.com
Two elements X (atomic mass=50) and Y (atomic mass=16) combine to give a compound having 32 Y Element X Atomic Mass The atomic weight is the average mass in atomic mass units of an element’s atoms. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The. Element X Atomic Mass.
From www.gkseries.com
An element X has mass number 40 and contains 21 neutrons in its atom. To which group of the Element X Atomic Mass It is simple to learn how to calculate the atomic mass of. The atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Elements usually have more than one isotope; Access detailed info on all elements: This atomic mass calculator shows you how to find the atomic mass of an atom. Element X Atomic Mass.