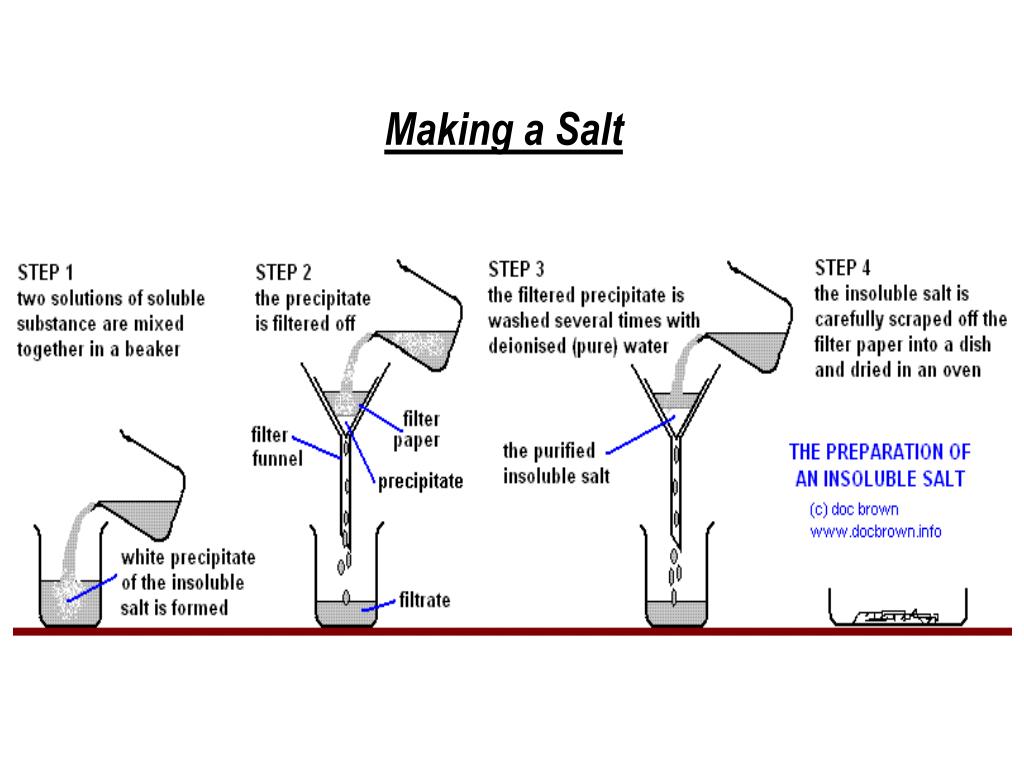
Silver Salt Method For Acids . Insoluble salts are made by precipitation reactions. The principle of this method is based on the. Adding acid to a solid metal, insoluble base or insoluble carbonate. Titration must be used if the reactants are soluble. The silver salt method is a chemical method of detection of organic acids. Soluble salts can be made by reacting acids with soluble or insoluble reactants. In most cases, you can add an excess of a solid metal. Diagram showing the preparation of soluble salts. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. There are two completely different experimental ways of doing this. A student uses this method to prepare a sample of silver chloride. Indicators are used to determine whether a. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis.
from www.slideserve.com
The principle of this method is based on the. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Indicators are used to determine whether a. Adding acid to a solid metal, insoluble base or insoluble carbonate. There are two completely different experimental ways of doing this. Insoluble salts are made by precipitation reactions. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. The silver salt method is a chemical method of detection of organic acids.
PPT Making a Salt PowerPoint Presentation, free download ID5368447
Silver Salt Method For Acids Insoluble salts are made by precipitation reactions. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Indicators are used to determine whether a. In most cases, you can add an excess of a solid metal. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Titration must be used if the reactants are soluble. There are two completely different experimental ways of doing this. The principle of this method is based on the. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. The silver salt method is a chemical method of detection of organic acids. A student uses this method to prepare a sample of silver chloride. Diagram showing the preparation of soluble salts. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. Insoluble salts are made by precipitation reactions. Adding acid to a solid metal, insoluble base or insoluble carbonate.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Silver Salt Method For Acids Soluble salts can be made by reacting acids with soluble or insoluble reactants. The silver salt method is a chemical method of detection of organic acids. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble. Silver Salt Method For Acids.
From www.youtube.com
Silver salt method for molecular determination of organic acids YouTube Silver Salt Method For Acids Diagram showing the preparation of soluble salts. The silver salt method is a chemical method of detection of organic acids. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Indicators are used to determine whether a. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or. Silver Salt Method For Acids.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Silver Salt Method For Acids Soluble salts can be made by reacting acids with soluble or insoluble reactants. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. The principle of this method is based on the. Insoluble salts are made by precipitation reactions. There are two completely different experimental. Silver Salt Method For Acids.
From fphoto.photoshelter.com
precipitate silver chloride chemistry reaction Fundamental Silver Salt Method For Acids Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Titration must be used if the reactants are soluble. In most cases, you can add an excess of a solid metal. The principle of this method is based on the. Soluble salts can be made. Silver Salt Method For Acids.
From www.youtube.com
Silver Salt MethodEquivalent Mass of Organic Acids YouTube Silver Salt Method For Acids Soluble salts can be made by reacting acids with soluble or insoluble reactants. The silver salt method is a chemical method of detection of organic acids. The principle of this method is based on the. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Most common chlorides (except lead(ii) and. Silver Salt Method For Acids.
From revisechemistry.uk
Making Salts AQA Required Practical revisechemistry.uk Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. There are two completely different experimental ways of doing this. A student uses this method to prepare a sample of silver chloride. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the. Silver Salt Method For Acids.
From www.researchgate.net
Preparation of Ag NPs from silver salt reduction with Norrish type I Silver Salt Method For Acids Soluble salts can be made by reacting acids with soluble or insoluble reactants. A student uses this method to prepare a sample of silver chloride. In most cases, you can add an excess of a solid metal. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such. Silver Salt Method For Acids.
From www.chegg.com
Solved What salt is formed in the reaction of silver Silver Salt Method For Acids Adding acid to a solid metal, insoluble base or insoluble carbonate. Diagram showing the preparation of soluble salts. There are two completely different experimental ways of doing this. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Soluble salts can be made by reacting. Silver Salt Method For Acids.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Silver Salt Method For Acids Adding acid to a solid metal, insoluble base or insoluble carbonate. A student uses this method to prepare a sample of silver chloride. Indicators are used to determine whether a. Insoluble salts are made by precipitation reactions. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. There are two completely different experimental ways. Silver Salt Method For Acids.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Silver Salt Method For Acids In most cases, you can add an excess of a solid metal. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Insoluble salts are made. Silver Salt Method For Acids.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID813901 Silver Salt Method For Acids Diagram showing the preparation of soluble salts. Insoluble salts are made by precipitation reactions. The principle of this method is based on the. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. There are two completely different experimental ways of doing this. In most cases, you can. Silver Salt Method For Acids.
From www.toppr.com
0.76g of a silver salt of a dibasic acid on ignition gave 0.54g of Silver Salt Method For Acids Titration must be used if the reactants are soluble. The silver salt method is a chemical method of detection of organic acids. There are two completely different experimental ways of doing this. Indicators are used to determine whether a. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble. Silver Salt Method For Acids.
From www.dreamstime.com
A Table of Silver Compounds Colors Stock Vector Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. A student uses this method to prepare a sample of silver chloride. There are two completely different experimental ways of doing this. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Insoluble salts are made by precipitation. Silver Salt Method For Acids.
From www.thermofisher.com
Diethyldithiocarbamic acid, silver salt, 99, for analysis, Thermo Silver Salt Method For Acids Titration must be used if the reactants are soluble. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. There are two completely different experimental ways of doing this. In most cases, you can add an excess of a solid metal. A student uses this. Silver Salt Method For Acids.
From www.vrogue.co
An Organic Diabasic Acid A Gave The Following On Anal vrogue.co Silver Salt Method For Acids In most cases, you can add an excess of a solid metal. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Diagram showing the preparation of soluble salts. There are two. Silver Salt Method For Acids.
From mavink.com
Titration Reaction Silver Salt Method For Acids Soluble salts can be made by reacting acids with soluble or insoluble reactants. Insoluble salts are made by precipitation reactions. Titration must be used if the reactants are soluble. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. Solutions of silver nitrate and potassium. Silver Salt Method For Acids.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Indicators are used to determine whether a. Diagram showing the preparation of soluble salts. Titration must be used if the reactants are soluble. Insoluble salts are made by precipitation reactions. Soluble salts can be made by reacting acids with soluble or. Silver Salt Method For Acids.
From www.researchgate.net
Silver salt screening for nucleophilic substitution reactions of Silver Salt Method For Acids Diagram showing the preparation of soluble salts. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Adding acid to a solid metal, insoluble base or insoluble carbonate. In most cases, you can add an excess of a solid metal. Describe. Silver Salt Method For Acids.
From www.researchgate.net
Silver salt screening for nucleophilic substitution reactions of Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. In most cases, you can add an excess of a solid metal. A student uses this method to prepare a sample of silver chloride. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Solutions of silver nitrate. Silver Salt Method For Acids.
From www.nagwa.com
Question Video Identifying the Salt Produced from an Acid and Metal Silver Salt Method For Acids Insoluble salts are made by precipitation reactions. Titration must be used if the reactants are soluble. The silver salt method is a chemical method of detection of organic acids. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Adding acid to a solid metal, insoluble base or insoluble carbonate. Diagram. Silver Salt Method For Acids.
From www.slideserve.com
PPT Making a Salt PowerPoint Presentation, free download ID5368447 Silver Salt Method For Acids Titration must be used if the reactants are soluble. The principle of this method is based on the. The silver salt method is a chemical method of detection of organic acids. Diagram showing the preparation of soluble salts. Indicators are used to determine whether a. Insoluble salts are made by precipitation reactions. Adding acid to a solid metal, insoluble base. Silver Salt Method For Acids.
From chem.libretexts.org
7.5 Precipitation Reactions Chemistry LibreTexts Silver Salt Method For Acids Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. The principle of this method is based on the. Indicators are used to determine whether a. The silver salt method is a chemical method of detection of organic acids. Adding acid to a solid metal,. Silver Salt Method For Acids.
From www.youtube.com
Silver salt Method for the calculation of Molecular mass of Organic Silver Salt Method For Acids A student uses this method to prepare a sample of silver chloride. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Diagram showing the preparation of soluble salts. The silver salt method is a chemical method of detection of organic acids. The principle of this method is based on the.. Silver Salt Method For Acids.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Silver Salt Method For Acids The silver salt method is a chemical method of detection of organic acids. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. The principle of this method is based on the. Soluble salts can be made by reacting acids with soluble or insoluble reactants.. Silver Salt Method For Acids.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. A student uses this method to prepare a sample of silver chloride. Insoluble salts are made by precipitation reactions. There are two completely different experimental. Silver Salt Method For Acids.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Silver Salt Method For Acids Titration must be used if the reactants are soluble. Insoluble salts are made by precipitation reactions. There are two completely different experimental ways of doing this. Adding acid to a solid metal, insoluble base or insoluble carbonate. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. A student uses this method to prepare. Silver Salt Method For Acids.
From www.vrogue.co
Silver Nitrate Agno3 Reacts With Hydrochloric Acid Hc vrogue.co Silver Salt Method For Acids A student uses this method to prepare a sample of silver chloride. Indicators are used to determine whether a. The silver salt method is a chemical method of detection of organic acids. Titration must be used if the reactants are soluble. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including.. Silver Salt Method For Acids.
From pubs.acs.org
Feasibility of Using a Electrochemical Cell for Silver Salt Method For Acids Indicators are used to determine whether a. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. Insoluble salts are made by precipitation reactions. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. There are two completely different experimental ways of doing this. The. Silver Salt Method For Acids.
From www.savemyexams.co.uk
Preparing Insoluble Salts (7.2.2) CIE IGCSE Chemistry Revision Notes Silver Salt Method For Acids Adding acid to a solid metal, insoluble base or insoluble carbonate. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. The. Silver Salt Method For Acids.
From dokumen.tips
(PDF) Antimicrobial and Hemostatic Effects of Silver Salts of Poly Silver Salt Method For Acids Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. There are two completely different experimental ways of doing this. In most cases, you can add an excess of a solid metal. Diagram showing the preparation of soluble salts. Soluble salts can be made by reacting acids with soluble or insoluble. Silver Salt Method For Acids.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID813901 Silver Salt Method For Acids Indicators are used to determine whether a. Adding acid to a solid metal, insoluble base or insoluble carbonate. Describe the general rules of solubility for common salts to include nitrates, chlorides (including silver and lead), sulfates (including. Diagram showing the preparation of soluble salts. In most cases, you can add an excess of a solid metal. A student uses this. Silver Salt Method For Acids.
From www.youtube.com
Making Salts From Acids & Metal Carbonates (GCSE Chemistry) YouTube Silver Salt Method For Acids Diagram showing the preparation of soluble salts. A student uses this method to prepare a sample of silver chloride. Insoluble salts are made by precipitation reactions. Most common chlorides (except lead(ii) and silver chlorides) soluble salts can be made by reacting acids with insoluble reactants or soluble reactants such as alkalis. There are two completely different experimental ways of doing. Silver Salt Method For Acids.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation, free Silver Salt Method For Acids Indicators are used to determine whether a. A student uses this method to prepare a sample of silver chloride. Adding acid to a solid metal, insoluble base or insoluble carbonate. Solutions of silver nitrate and potassium chloride react together to make the insoluble salt, silver chloride. There are two completely different experimental ways of doing this. Insoluble salts are made. Silver Salt Method For Acids.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru Silver Salt Method For Acids There are two completely different experimental ways of doing this. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. Adding acid to a solid metal, insoluble base or insoluble carbonate. Diagram showing the preparation of soluble salts. In most cases, you can add an excess of a. Silver Salt Method For Acids.
From www.chegg.com
Solved What salt is formed in the reaction of silver with Silver Salt Method For Acids The principle of this method is based on the. Diagram showing the preparation of soluble salts. The silver salt method is a chemical method of detection of organic acids. In most cases, you can add an excess of a solid metal. Soluble salts can be made by reacting acids with soluble or insoluble reactants. A student uses this method to. Silver Salt Method For Acids.