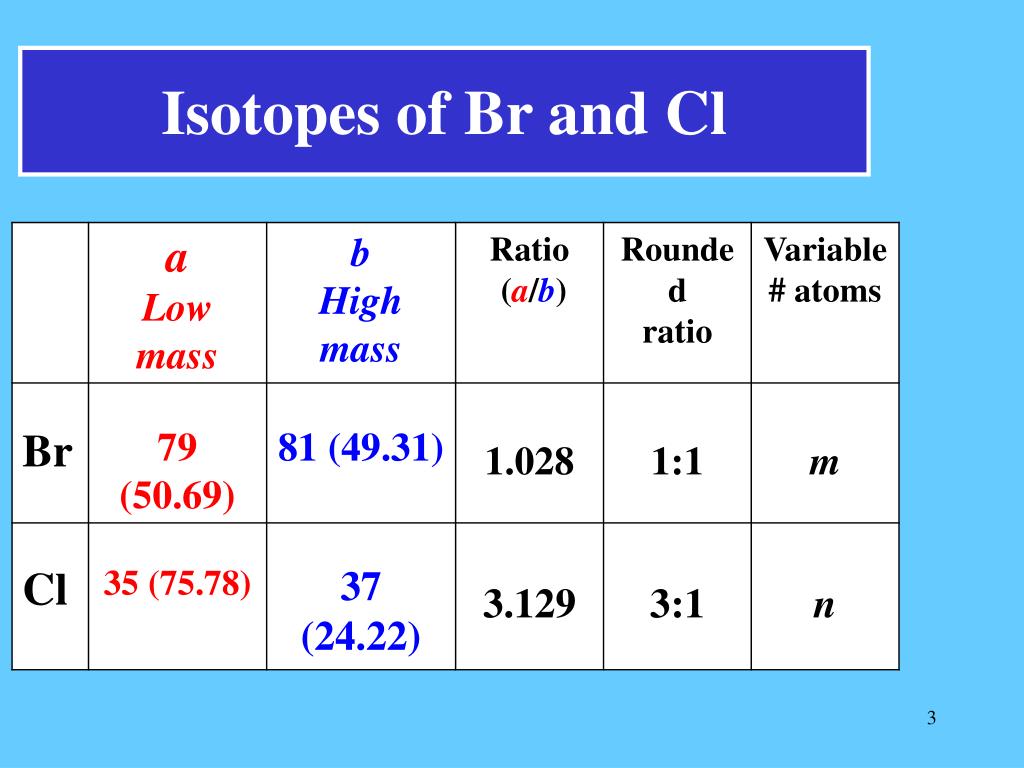
Chlorine Isotope Calculation . For compounds of chlorine and. There are two stable isotopes , 35 cl. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. And their natural abundances are 75.78% and 24.22%, respectively. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. The following diagrams show the isotopes of. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. The molecular ion containing the 35. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Their atomic masses are 34.96885 amu and 36.96590 amu. In this case, you have the.
from www.slideserve.com
The following diagrams show the isotopes of. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. And their natural abundances are 75.78% and 24.22%, respectively. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. There are two stable isotopes , 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The molecular ion containing the 35. Their atomic masses are 34.96885 amu and 36.96590 amu. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. For compounds of chlorine and.
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Containing Multiple
Chlorine Isotope Calculation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. For compounds of chlorine and. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The molecular ion containing the 35. Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The following diagrams show the isotopes of. Their atomic masses are 34.96885 amu and 36.96590 amu. In this case, you have the. There are two stable isotopes , 35 cl. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. And their natural abundances are 75.78% and 24.22%, respectively. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry Chlorine Isotope Calculation The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine has two isotopes, with 75.53% being 35. Chlorine Isotope Calculation.
From mavink.com
Relative Atomic Mass Formula Isotopes Chlorine Isotope Calculation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? It shows how you can find out the masses and relative abundances of. Chlorine Isotope Calculation.
From www.showme.com
Calculating RAM of Cl Science, Chemistry, Isotopes, AS Level ShowMe Chlorine Isotope Calculation 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. There are two stable isotopes , 35 cl. And their natural abundances are 75.78% and 24.22%, respectively. It shows how you can find out the masses and relative abundances of the. Chlorine Isotope Calculation.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Calculation In this case, you have the. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Their atomic masses are 34.96885 amu and 36.96590 amu. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the. Chlorine Isotope Calculation.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 and... Course Hero Chlorine Isotope Calculation Their atomic masses are 34.96885 amu and 36.96590 amu. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The molecular ion containing the 35. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of. Chlorine Isotope Calculation.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Chlorine Isotope Calculation Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The molecular ion containing the 35. For compounds of chlorine and. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. The. Chlorine Isotope Calculation.
From www.numerade.com
tzvb question 3 homework answered cl cl cl while the image above shows you only 3 isotopes of Chlorine Isotope Calculation It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. And their natural abundances are 75.78% and 24.22%, respectively. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations.. Chlorine Isotope Calculation.
From www.youtube.com
CHEMISTRY 101 Calculating mass of an isotope in a sample YouTube Chlorine Isotope Calculation It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. And their natural abundances are 75.78% and 24.22%, respectively. There are two stable isotopes , 35 cl. Use the atomic masses of each of the two isotopes of. Chlorine Isotope Calculation.
From www.answersarena.com
[Solved] While the image above shows you only 3 isotopes o Chlorine Isotope Calculation There are two stable isotopes , 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. Chlorine has two isotopes, with. Chlorine Isotope Calculation.
From www.vrogue.co
Education Information How To Calculate Average Atomic vrogue.co Chlorine Isotope Calculation Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The following diagrams show the isotopes of. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 12 rows only the mass of the most abundant. Chlorine Isotope Calculation.
From www.chegg.com
Solved Please use the following table to calculate the Chlorine Isotope Calculation It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. And their natural abundances are 75.78% and 24.22%, respectively. There are two stable isotopes , 35 cl. Chlorine has two isotopes, with 75.53% being 35 cl with an. Chlorine Isotope Calculation.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Isotope Calculation For compounds of chlorine and. Their atomic masses are 34.96885 amu and 36.96590 amu. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. The molecular ion containing the 35. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used. Chlorine Isotope Calculation.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Chlorine Isotope Calculation The following diagrams show the isotopes of. In this case, you have the. And their natural abundances are 75.78% and 24.22%, respectively. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The molecular ion containing the 35. For compounds of chlorine and. It shows how you. Chlorine Isotope Calculation.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotope Calculation There are two stable isotopes , 35 cl. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. For compounds of chlorine and. In this case, you have the. Their atomic masses are 34.96885 amu and 36.96590 amu. The following diagrams show the isotopes of. Use the atomic masses of each of the two isotopes of chlorine along with their. Chlorine Isotope Calculation.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID2050967 Chlorine Isotope Calculation The molecular ion containing the 35. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. Use the atomic masses of each. Chlorine Isotope Calculation.
From www.chegg.com
Solved There are two naturally occurring isotopes of Chlorine Isotope Calculation For compounds of chlorine and. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. The following diagrams show the isotopes of. And their natural abundances are. Chlorine Isotope Calculation.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Containing Multiple Chlorine Isotope Calculation And their natural abundances are 75.78% and 24.22%, respectively. In this case, you have the. The molecular ion containing the 35. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Their atomic masses are 34.96885 amu and. Chlorine Isotope Calculation.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine Chlorine Isotope Calculation And their natural abundances are 75.78% and 24.22%, respectively. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. In this case, you have the. For compounds of chlorine and. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use. Chlorine Isotope Calculation.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Calculation Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. The molecular ion containing the 35. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52. Chlorine Isotope Calculation.
From www.youtube.com
Calculate the atomic mass (average) of chlorine using the following data YouTube Chlorine Isotope Calculation 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. The two stable isotopes of chlorine are ³⁵cl and. Chlorine Isotope Calculation.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Photo Library Chlorine Isotope Calculation Their atomic masses are 34.96885 amu and 36.96590 amu. There are two stable isotopes , 35 cl. In this case, you have the. Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The following diagrams show the isotopes of. 53 rows chlorine (17 cl) has 25 isotopes, ranging. Chlorine Isotope Calculation.
From www.youtube.com
Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 31. Calculate the avg Chlorine Isotope Calculation In this case, you have the. The molecular ion containing the 35. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine has two isotopes, with 75.53%. Chlorine Isotope Calculation.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic weight 35 and 37.What is the Chlorine Isotope Calculation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Their atomic masses are 34.96885 amu and 36.96590 amu. The molecular ion containing the 35. For compounds of chlorine and. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to. Chlorine Isotope Calculation.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry Chlorine Isotope Calculation There are two stable isotopes , 35 cl. In this case, you have the. For compounds of chlorine and. And their natural abundances are 75.78% and 24.22%, respectively. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element.. Chlorine Isotope Calculation.
From questions-in.kunduz.com
11 2. Chlorine has isotopes 35 Cl and Cl.... Physical Chemistry Chlorine Isotope Calculation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. The molecular ion containing the 35. For compounds of chlorine and. Their atomic. Chlorine Isotope Calculation.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Calculation Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The molecular ion containing the 35. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Their atomic masses are 34.96885 amu and 36.96590 amu. And their natural abundances are. Chlorine Isotope Calculation.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID5850956 Chlorine Isotope Calculation The molecular ion containing the 35. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. For compounds of chlorine and. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. The following diagrams show the isotopes of.. Chlorine Isotope Calculation.
From www.slideserve.com
PPT Isotopes PowerPoint Presentation, free download ID4854680 Chlorine Isotope Calculation The following diagrams show the isotopes of. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. The molecular ion containing the 35. There are two stable isotopes , 35 cl. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. In this case, you have the. Use the atomic masses. Chlorine Isotope Calculation.
From askfilo.com
Problem 4.2 The two natural isotopes of chlorine, viz. 1735 Cl and 1737.. Chlorine Isotope Calculation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two stable isotopes , 35 cl. Their atomic masses are 34.96885 amu and 36.96590 amu. It shows how you can find out the masses and relative abundances of the various isotopes of the element and. Chlorine Isotope Calculation.
From www.slideserve.com
PPT 2.1 Elements and Symbols PowerPoint Presentation, free download ID6998787 Chlorine Isotope Calculation For compounds of chlorine and. Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The following diagrams show the isotopes of. The two stable isotopes of chlorine are ³⁵cl and ³⁷cl. The molecular ion containing the 35. And their natural abundances are 75.78% and 24.22%, respectively. In these. Chlorine Isotope Calculation.
From brainly.in
If chlorine atom is available in the form of two isotopes 35cl 75and 37cl25 calculate the Chlorine Isotope Calculation Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. The following diagrams show the isotopes of. In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? 53 rows. Chlorine Isotope Calculation.
From www.numerade.com
SOLVED Title Isotopes of Chlorine and Average Atomic Mass Calculation While the image above Chlorine Isotope Calculation Their atomic masses are 34.96885 amu and 36.96590 amu. For compounds of chlorine and. Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. In these. Chlorine Isotope Calculation.
From www.toppr.com
The isotopes of chlorine with mass number 35 and 37 exist in the ratio of Chlorine Isotope Calculation 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Chlorine has two isotopes, with 75.53% being 35 cl. Chlorine Isotope Calculation.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass is 35.5 then the ratio of Chlorine Isotope Calculation In these lessons, we will learn about isotopes, isotope notation, atomic mass unit (amu), and how to calculate the atomic mass of an element. In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? For compounds of chlorine and. The. Chlorine Isotope Calculation.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Elements and Chemistry Chlorine Isotope Calculation There are two stable isotopes , 35 cl. In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The following diagrams show the isotopes of. Use the atomic masses of each of the two isotopes of chlorine along with their. Chlorine Isotope Calculation.