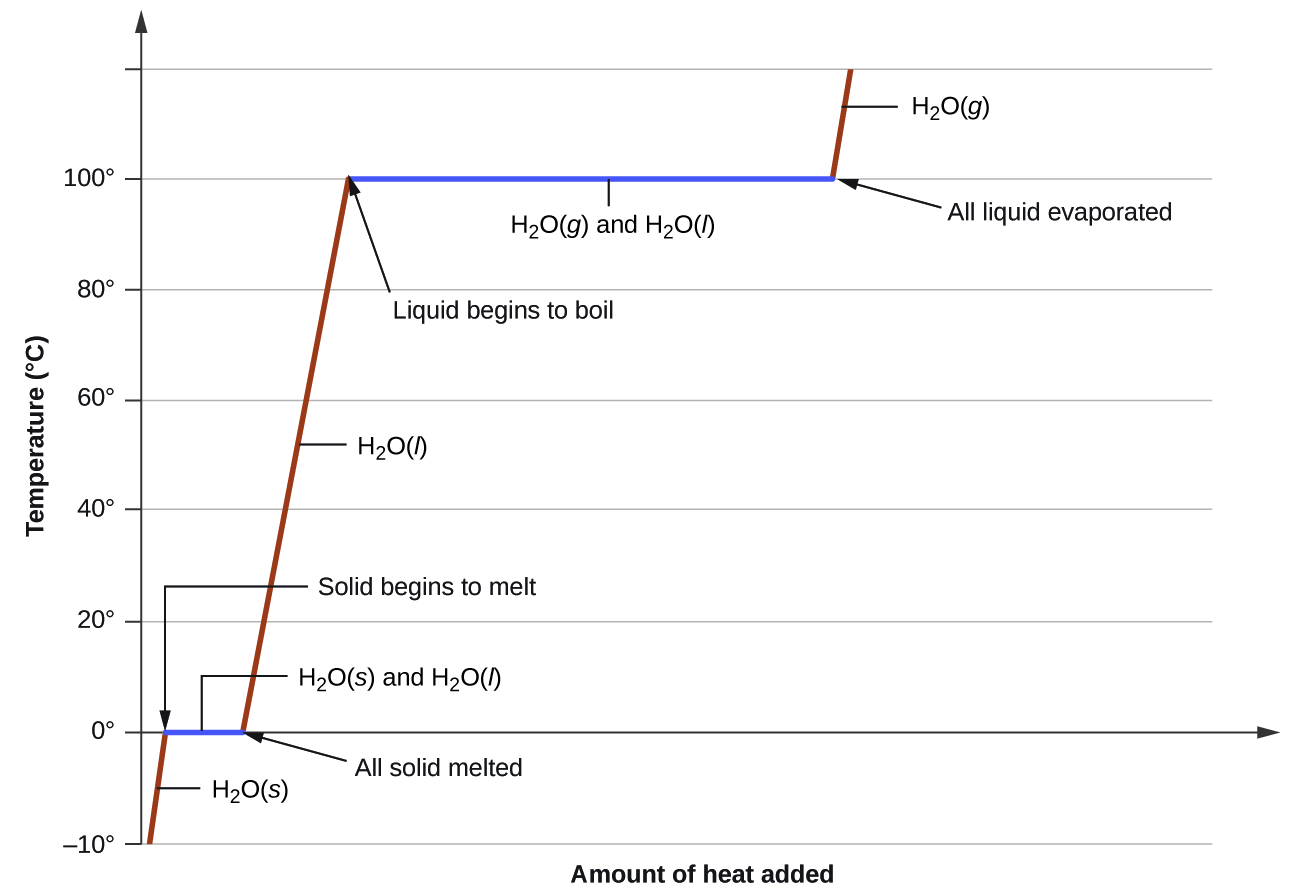
Heating Curve Segments . They show how the temperature changes as a substance is. Heating curves relate temperature changes to phase. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Cooling curves are the opposite. For cooling curves, we start with the gas phase and. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. For heating curves, we start with a solid and add heat energy. Heating curves show how the temperature changes as a substance is heated up.
from chem.libretexts.org
For cooling curves, we start with the gas phase and. Cooling curves are the opposite. Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. They show how the temperature changes as a substance is. Heating curves show how the temperature changes as a substance is heated up. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). For heating curves, we start with a solid and add heat energy. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:
8.1 Heating Curves and Phase Changes Chemistry LibreTexts
Heating Curve Segments For cooling curves, we start with the gas phase and. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. They show how the temperature changes as a substance is. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Heating curves relate temperature changes to phase. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Cooling curves are the opposite. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves show how the temperature changes as a substance is heated up. For cooling curves, we start with the gas phase and. For heating curves, we start with a solid and add heat energy.
From www.researchgate.net
4 DSC heating and cooling curves for asreceived Neo Sentalloy Heating Curve Segments They show how the temperature changes as a substance is. Heating curves relate temperature changes to phase. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Cooling curves are the opposite. For cooling curves, we start with the gas phase and. This plot of temperature shows what happens. Heating Curve Segments.
From hrsbstaff.ednet.ns.ca
Science 10 Heating Curve Segments For heating curves, we start with a solid and add heat energy. Cooling curves are the opposite. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. This plot of. Heating Curve Segments.
From quizizz.com
Heating and Cooling Curves Chemistry Quiz Quizizz Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. They show how the temperature changes as a substance is. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). This plot of temperature shows what happens to a 75 g sample of ice initially at. Heating Curve Segments.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Segments Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant. Heating Curve Segments.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Segments Cooling curves are the opposite. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. For cooling curves, we start with the gas phase and. They show how the temperature changes as a substance is. The curve is divided into distinct segments, each corresponding to a. Heating Curve Segments.
From www.doubtnut.com
The temperaturetime graph below shows the heating curve for pure wax Heating Curve Segments The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Heating curves show how the temperature changes as a substance is heated up. For cooling curves, we start with the gas phase and. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate).. Heating Curve Segments.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes to phase. Cooling curves are the opposite. For cooling curves, we start with the gas phase and. They. Heating Curve Segments.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating Curve Segments For heating curves, we start with a solid and add heat energy. Cooling curves are the opposite. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Heating curves show how the temperature changes as a substance is heated up. For cooling curves, we start with the gas phase. Heating Curve Segments.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Heating Curve Segments For heating curves, we start with a solid and add heat energy. Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. They show how the temperature changes as a substance is. For cooling curves, we start with the. Heating Curve Segments.
From www.albert.io
Heating Curve and Specific Heat Chemistry Practice Albert Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). They show how the temperature changes as a substance is. Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Heating Curve Segments.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Cooling curves are the opposite. They show how the temperature changes as a substance is. Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat. Heating Curve Segments.
From preparatorychemistry.com
Heating Curve Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). This plot of temperature shows what happens to a 75. Heating Curve Segments.
From heatinggondon.blogspot.com
Heating A Heating Curve Heating Curve Segments The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Cooling curves are the opposite. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). For cooling curves, we start with the gas phase and. Plots of the temperature of a substance versus. Heating Curve Segments.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Segments They show how the temperature changes as a substance is. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes to phase. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. This plot. Heating Curve Segments.
From www.doubtnut.com
Which segment of the heating curve obtained at constant pressure corre Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). For heating curves, we start with a solid and add heat energy. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. For cooling curves,. Heating Curve Segments.
From www.slideserve.com
PPT Freezing and Boiling Point Graph aka Phase Change Diagram or Heating Curve Segments Cooling curves are the opposite. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. This plot of. Heating Curve Segments.
From www.doubtnut.com
The temperaturetime graph below shows the heating curve for pure wax Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). They show how the temperature changes as a substance is. Heating curves relate temperature changes to phase. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. This plot of temperature shows what. Heating Curve Segments.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heating Curve Segments For heating curves, we start with a solid and add heat energy. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: For cooling curves, we start with the gas phase and. A heating curve of a substance shows the relationship of. Heating Curve Segments.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Segments Heating curves relate temperature changes to phase. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Heating curves show how the temperature changes as a substance is heated up. The curve. Heating Curve Segments.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Segments They show how the temperature changes as a substance is. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). For cooling curves, we start. Heating Curve Segments.
From quizizz.com
Heating Curve Graphs Chemistry Quizizz Heating Curve Segments For cooling curves, we start with the gas phase and. Cooling curves are the opposite. Heating curves relate temperature changes to phase. They show how the temperature changes as a substance is. For heating curves, we start with a solid and add heat energy. This plot of temperature shows what happens to a 75 g sample of ice initially at. Heating Curve Segments.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). They show how the temperature changes as a substance is. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes. Heating Curve Segments.
From www.numerade.com
SOLVED Bas liquid and gas 2 solid and liquid liquid solid Heat added Heating Curve Segments Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). For cooling curves, we start with the gas phase and. This plot of temperature shows. Heating Curve Segments.
From www.researchgate.net
Heating (curve segments a) and cooling (segments b and c) recorded at a Heating Curve Segments The curve is divided into distinct segments, each corresponding to a specific phase of the substance. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat. Heating Curve Segments.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating Curve Segments This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Cooling curves are the opposite. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Heating curves show how the temperature changes as a substance is. Heating Curve Segments.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Segments For heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Heating curves relate temperature changes to phase. This plot of temperature shows what happens to a 75 g sample of ice initially. Heating Curve Segments.
From www.animalia-life.club
A Heating Curve Illustrates Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: They show how the temperature changes as. Heating Curve Segments.
From brainly.com
Consider the heating curve of H20 and line segments A, B, and C Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes to phase. Cooling curves are the opposite. This plot of temperature shows what happens to a 75 g. Heating Curve Segments.
From www.pinterest.com
Pin by Kristen Alexis on MCAT in 2023 Chemistry lessons, Chemistry Heating Curve Segments A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). They show how the temperature changes as a substance is. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. For cooling curves, we start. Heating Curve Segments.
From curiophysics.com
Heating Curve » Curio Physics Heating Curve Segments Heating curves relate temperature changes to phase. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. They show how the temperature changes as a substance is. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). The curve is divided into. Heating Curve Segments.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. Heating curves relate temperature changes to phase. For heating curves, we start with a solid and add heat energy. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. They show how the. Heating Curve Segments.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. They show how the temperature changes as a substance is. For heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and. Heating curves relate temperature changes to phase. The curve is divided into distinct segments, each. Heating Curve Segments.
From www.chegg.com
Solved Use the heating curve provided to answer the Heating Curve Segments Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. They show how the temperature changes as a substance is. Heating curves relate temperature changes to phase. For cooling curves, we start with the gas phase and. Heating curves show how the temperature changes as a. Heating Curve Segments.
From www.numerade.com
SOLVED Which line segment in the graph shown below represents the heat Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. Heating Curve Segments.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Segments Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Plots of the temperature of a substance versus heat. Heating Curve Segments.