Diluted With Water To 5 Acidity . If diluted to 5% acetic acid, which is the concentration of acetic acid. It says, apple cider vinegar diluted with water to 5% acidity. This is the percentage of acid in it and not the. Suppose vinegar is 100% acetic acid. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. Its ph is around 4.76. Solution as in previous examples, the definition of. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. It says that its acidity is 5%. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar
from www.vecteezy.com
Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Suppose vinegar is 100% acetic acid. It says that its acidity is 5%. This is the percentage of acid in it and not the. Solution as in previous examples, the definition of. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar It says, apple cider vinegar diluted with water to 5% acidity. Its ph is around 4.76.
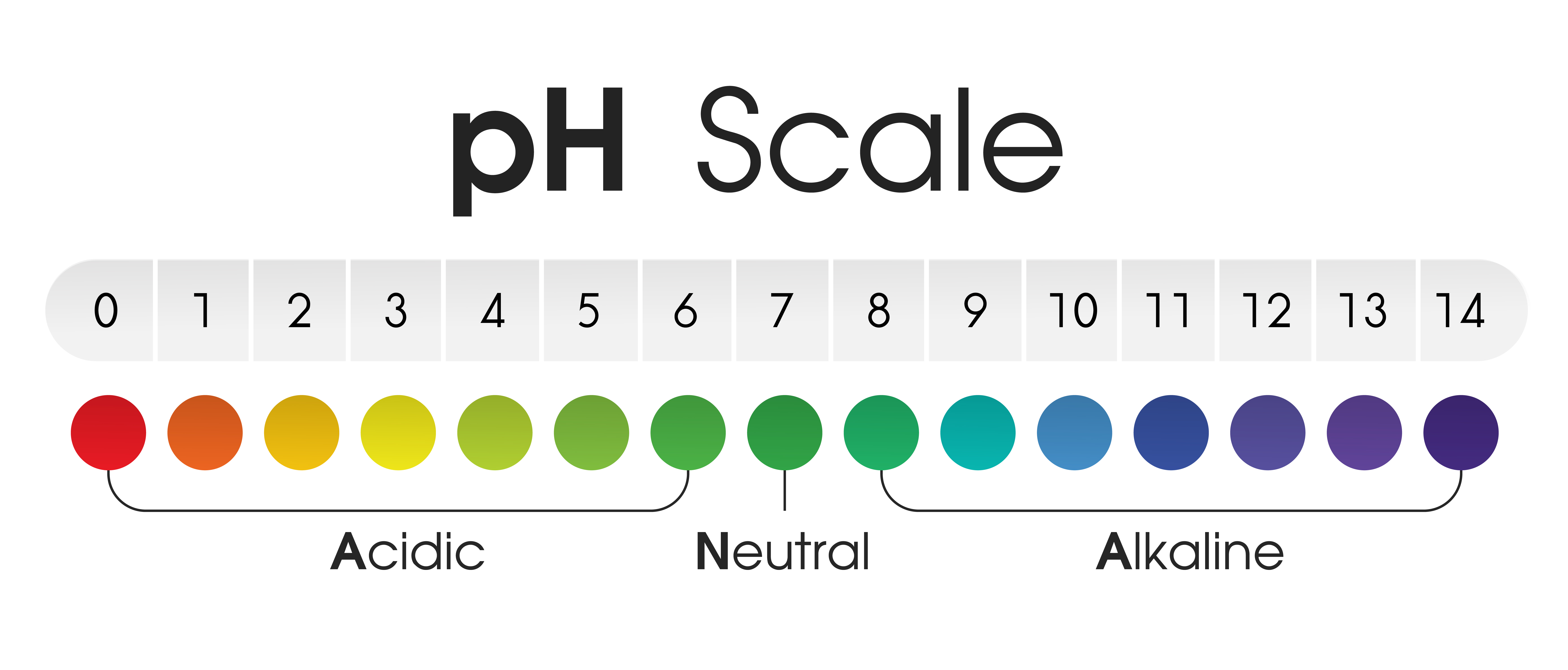
The chart shows the Acidic Neutral and Alkaline pH of various liquids and solvents. Vector
Diluted With Water To 5 Acidity Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Suppose vinegar is 100% acetic acid. It says, apple cider vinegar diluted with water to 5% acidity. Its ph is around 4.76. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. This is the percentage of acid in it and not the. It says that its acidity is 5%. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. If diluted to 5% acetic acid, which is the concentration of acetic acid. Solution as in previous examples, the definition of. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar
From www.drdarrinlew.us
Acidity And Alkalinity Water Quality Dr. Darrin Lew Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can. Diluted With Water To 5 Acidity.
From www.scienceabc.com
What Is Gastric Acid (Stomach Acid)? Is It Diluted When We Drink Water? Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. It says that its acidity is 5%. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Its ph is around 4.76. In modern home. Diluted With Water To 5 Acidity.
From www.numerade.com
SOLVED Approximately 6 mL of concentrated perchloric acid (72) was transferred to a bottle and Diluted With Water To 5 Acidity Suppose vinegar is 100% acetic acid. It says that its acidity is 5%. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. For 25% acidity to 5% acidity combine 4 parts water and 1 part. Diluted With Water To 5 Acidity.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Diluted With Water To 5 Acidity In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. It says that its acidity is 5%. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. Its ph is around 4.76. Solution as in previous. Diluted With Water To 5 Acidity.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Diluted With Water To 5 Acidity If diluted to 5% acetic acid, which is the concentration of acetic acid. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Solution as in previous examples, the definition of. For 25% acidity to 5% acidity combine 4 parts water and 1 part. Diluted With Water To 5 Acidity.
From www.wou.edu
CH103 Chapter 8 Homeostasis and Cellular Function Chemistry Diluted With Water To 5 Acidity This is the percentage of acid in it and not the. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. If diluted to 5% acetic acid, which is the concentration of acetic acid. It says that its acidity is 5%. Before you perform the dilution. Diluted With Water To 5 Acidity.
From www.ucdavis.edu
Ocean Acidification UC Davis Diluted With Water To 5 Acidity If diluted to 5% acetic acid, which is the concentration of acetic acid. It says, apple cider vinegar diluted with water to 5% acidity. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Its ph is around 4.76. Suppose vinegar is 100% acetic. Diluted With Water To 5 Acidity.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Biological Supply Diluted With Water To 5 Acidity If diluted to 5% acetic acid, which is the concentration of acetic acid. Suppose vinegar is 100% acetic acid. Its ph is around 4.76. This is the percentage of acid in it and not the. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. It. Diluted With Water To 5 Acidity.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Diluted With Water To 5 Acidity This is the percentage of acid in it and not the. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. If diluted to 5% acetic acid, which is the concentration of acetic acid. Diluting acid with water is exothermic, so it’s easier to boil and. Diluted With Water To 5 Acidity.
From blendwell.blogspot.com
Blendwell Chemicals How to Dilute Cleaning Chemicals Diluted With Water To 5 Acidity The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. If diluted to 5% acetic acid, which is the concentration of acetic acid. Solution as in previous examples, the definition of. Suppose vinegar is 100% acetic acid. This is the percentage of acid in it and. Diluted With Water To 5 Acidity.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID1709572 Diluted With Water To 5 Acidity Suppose vinegar is 100% acetic acid. Its ph is around 4.76. It says that its acidity is 5%. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. It says, apple cider vinegar diluted with water to 5% acidity. If diluted to 5% acetic. Diluted With Water To 5 Acidity.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Diluted With Water To 5 Acidity Suppose vinegar is 100% acetic acid. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. It says, apple cider. Diluted With Water To 5 Acidity.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Art of Science Diluted With Water To 5 Acidity Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar It says, apple cider vinegar diluted with water to 5%. Diluted With Water To 5 Acidity.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution YouTube Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. Its ph is around 4.76. Suppose vinegar is 100% acetic acid. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. It says that its acidity is 5%. Before you perform the dilution. Diluted With Water To 5 Acidity.
From www.youtube.com
Dilute Sulphuric Acid Test Chemistry Lab Manual YouTube Diluted With Water To 5 Acidity Solution as in previous examples, the definition of. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5%. Diluted With Water To 5 Acidity.
From www.vrogue.co
10 Examples Of Solutions vrogue.co Diluted With Water To 5 Acidity In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Suppose vinegar is 100% acetic acid. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat. Diluted With Water To 5 Acidity.
From www.vecteezy.com
The chart shows the Acidic Neutral and Alkaline pH of various liquids and solvents. Vector Diluted With Water To 5 Acidity Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Its ph is around 4.76. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. The label has. Diluted With Water To 5 Acidity.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID949751 Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. It says that its acidity is 5%. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. If diluted to 5% acetic acid, which is the concentration of acetic acid. Suppose vinegar is 100% acetic acid. For 25% acidity to 5%. Diluted With Water To 5 Acidity.
From www.youtube.com
pH of Dilute Solution (Example) YouTube Diluted With Water To 5 Acidity Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. Suppose vinegar is 100% acetic acid. Solution as in previous examples, the definition of. This is the percentage of acid in it and not the. If diluted to 5% acetic acid, which is the concentration of acetic acid. In modern home canning,. Diluted With Water To 5 Acidity.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of moles of solute remains Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. It says that its acidity is 5%. Suppose vinegar is 100% acetic acid. Its ph is around 4.76. This is the percentage of acid in it and not the. If diluted to 5% acetic acid, which is the concentration of acetic acid. The label has a picture of a. Diluted With Water To 5 Acidity.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Diluted With Water To 5 Acidity Suppose vinegar is 100% acetic acid. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written. Diluted With Water To 5 Acidity.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Diluted With Water To 5 Acidity Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. This is the percentage of acid in it and not the. Its ph is around 4.76. For 25% acidity to 5% acidity combine 4 parts water. Diluted With Water To 5 Acidity.
From democracyunlimited.web.fc2.com
diluting acid with water Diluted With Water To 5 Acidity In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. This is the percentage of acid in it and not the. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because. Diluted With Water To 5 Acidity.
From study.com
How to Dilute a Strong Acid Solution to a Given pH Chemistry Diluted With Water To 5 Acidity Its ph is around 4.76. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. Solution as in previous examples, the definition of. This is the percentage of acid in it and not the. It says. Diluted With Water To 5 Acidity.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids Diluted With Water To 5 Acidity Its ph is around 4.76. Suppose vinegar is 100% acetic acid. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is. Diluted With Water To 5 Acidity.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Diluted With Water To 5 Acidity This is the percentage of acid in it and not the. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity. Diluted With Water To 5 Acidity.
From www.youtube.com
Acidity of Water Acidity Minerals Acidity & Total Acidity Environmental Pollution and Diluted With Water To 5 Acidity In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. If diluted to 5% acetic acid, which is the concentration of acetic acid. Solution as in previous examples, the definition of. It says that its acidity is 5%. Suppose vinegar is 100% acetic acid.. Diluted With Water To 5 Acidity.
From chem.libretexts.org
Acid Dissociation Constant Chemistry LibreTexts Diluted With Water To 5 Acidity It says that its acidity is 5%. Suppose vinegar is 100% acetic acid. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5%. Diluted With Water To 5 Acidity.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Diluted With Water To 5 Acidity Diluting acid with water is exothermic, so it’s easier to boil and splash water added to acid than acid added to water because water has a high heat capacity and can absorb a lot. This is the percentage of acid in it and not the. Before you perform the dilution itself, calculate the amount of water and acid needed for. Diluted With Water To 5 Acidity.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Diluted With Water To 5 Acidity In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. Diluting acid with water is exothermic, so it’s easier to. Diluted With Water To 5 Acidity.
From www.slideserve.com
PPT Acids and Alkalis PowerPoint Presentation, free download ID5037746 Diluted With Water To 5 Acidity Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. This is the percentage of acid in it and not the. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Diluting acid with water is. Diluted With Water To 5 Acidity.
From www.youtube.com
3. Strong, weak, dilute and concentrated acids (HSC chemistry) YouTube Diluted With Water To 5 Acidity Solution as in previous examples, the definition of. Its ph is around 4.76. The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. Before you perform the dilution itself, calculate the amount of water and acid needed for the desired concentration. Suppose vinegar is 100% acetic. Diluted With Water To 5 Acidity.
From www.toppr.com
The pH of HCl is 5. It is diluted by 1000 times. Its pH will be Diluted With Water To 5 Acidity It says, apple cider vinegar diluted with water to 5% acidity. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Solution as in previous examples, the definition of. Its ph is around 4.76. For 25% acidity to 5% acidity combine 4 parts water. Diluted With Water To 5 Acidity.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Diluted With Water To 5 Acidity If diluted to 5% acetic acid, which is the concentration of acetic acid. Its ph is around 4.76. In modern home canning, the accepted rule of thumb for safe vinegar / water pickling solutions is that the vinegar should be of at least 5%. Diluting acid with water is exothermic, so it’s easier to boil and splash water added to. Diluted With Water To 5 Acidity.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Diluted With Water To 5 Acidity For 25% acidity to 5% acidity combine 4 parts water and 1 part 25% acidity vinegar for 24% acidity to 5% acidity combine 19 parts water and 5 parts 24% acidity vinegar The label has a picture of a salad with the words “distilled white vinegar,” and, “reduced with water to 5% acidity,” written above it. Before you perform the. Diluted With Water To 5 Acidity.