Does Heat Increase Water Pressure . Yes, at constant density, the pressure increases as the temperature does: When you heat the water it expands, which does work against the surrounding pressure. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. For example, having water sealed at atmospheric. A large body of water, such as. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. At higher pressure, the expansion takes more work. Which means, that much heat is required to. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Most solids expand when heated,.
from ch301.cm.utexas.edu
The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. A large body of water, such as. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Most solids expand when heated,. At higher pressure, the expansion takes more work. For example, having water sealed at atmospheric. Which means, that much heat is required to. When you heat the water it expands, which does work against the surrounding pressure. Yes, at constant density, the pressure increases as the temperature does: Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$.
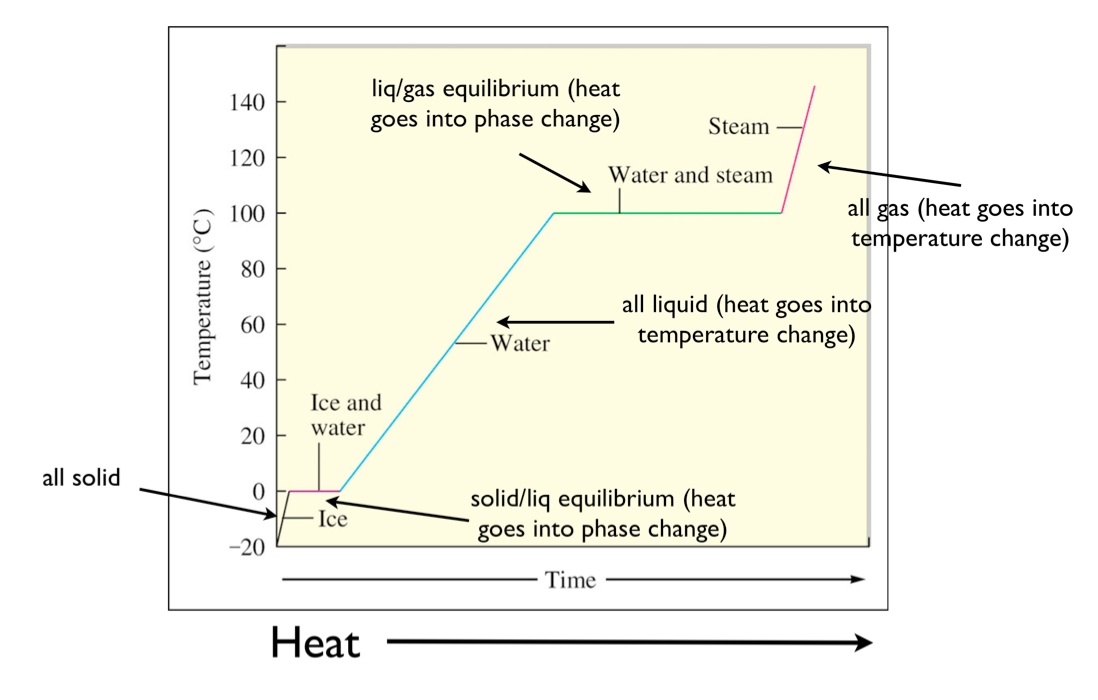
heating curve
Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. When you heat the water it expands, which does work against the surrounding pressure. At higher pressure, the expansion takes more work. Which means, that much heat is required to. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Yes, at constant density, the pressure increases as the temperature does: A large body of water, such as. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. For example, having water sealed at atmospheric. Most solids expand when heated,.
From techheaters.com
How To Increase Water Pressure With Tankless Water Heater? Does Heat Increase Water Pressure For example, having water sealed at atmospheric. When you heat the water it expands, which does work against the surrounding pressure. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. At higher pressure, the expansion takes more work. Which means, that much heat is required to. The reason is that water has a greater specific. Does Heat Increase Water Pressure.
From heaterguides.com
How To Increase Water Pressure With Tankless Water Heater? Does Heat Increase Water Pressure A large body of water, such as. At higher pressure, the expansion takes more work. When you heat the water it expands, which does work against the surrounding pressure. Most solids expand when heated,. For example, having water sealed at atmospheric. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there. Does Heat Increase Water Pressure.
From realestateinfoguide.com
10 Ways to Increase Home Water Pressure Real Estate Info Guide Does Heat Increase Water Pressure Yes, at constant density, the pressure increases as the temperature does: Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Pressure and temperature were fairly well. Does Heat Increase Water Pressure.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Heat Increase Water Pressure At higher pressure, the expansion takes more work. For example, having water sealed at atmospheric. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Keeping the volume exactly constant while increasing the temperature is not as simple as it may. Does Heat Increase Water Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download ID6717792 Does Heat Increase Water Pressure Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Which means, that much heat is required to. Most solids expand when heated,. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of. Does Heat Increase Water Pressure.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Does Heat Increase Water Pressure Most solids expand when heated,. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Which means, that much heat is required to. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Yes, at constant density,. Does Heat Increase Water Pressure.
From recipeprojectblog.com
How to Increase Water Pressure in Kitchen Sink? The Recipe Project Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Which means, that much heat is required to. Yes, at constant density, the pressure increases as the temperature does: A large body of water, such as. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Pressure and temperature. Does Heat Increase Water Pressure.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Chemistry Does Heat Increase Water Pressure For example, having water sealed at atmospheric. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. A large body of water, such as. The reason is that water has a greater specific heat than most common substances and. Does Heat Increase Water Pressure.
From www.scplumbing.co.uk
Pipe sizing a pitfall when upgrading your heating system SC Plumbing & Gas Tamworths best Does Heat Increase Water Pressure Which means, that much heat is required to. Most solids expand when heated,. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. A large body of water, such as. Pressure and temperature were fairly well understood in the. Does Heat Increase Water Pressure.
From beulacardwell.blogspot.com
how to increase water pressure in house with well Beula Cardwell Does Heat Increase Water Pressure For example, having water sealed at atmospheric. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Most solids expand when heated,. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm. Does Heat Increase Water Pressure.
From socratic.org
What is the relation between critical temperature and boiling point or vapor pressure? Socratic Does Heat Increase Water Pressure Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Most solids expand when heated,. For example, having water sealed at atmospheric. Yes, at. Does Heat Increase Water Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Heat Increase Water Pressure Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Yes, at constant density, the pressure increases as the temperature does: When you heat the water it expands, which does work against the surrounding pressure. Most solids expand when heated,. At higher pressure, the expansion takes more work. Pressure and temperature were fairly well understood in. Does Heat Increase Water Pressure.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Yes, at constant density, the pressure increases as the temperature does: The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. At higher pressure, the expansion takes. Does Heat Increase Water Pressure.
From www.researchgate.net
The effect of pressure on the thermophysical properties of water. (A)... Download Scientific Does Heat Increase Water Pressure Most solids expand when heated,. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. A large body of water, such as. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. When you heat the water it expands, which does work against the surrounding pressure. Pressure and temperature. Does Heat Increase Water Pressure.
From accurateleaklocators.com
How to Change Water Pressure in Your Home Accurate Leak Locators Does Heat Increase Water Pressure Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Yes, at constant density, the pressure increases as the temperature does: Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. At higher pressure, the expansion takes more work. Most solids expand when heated,. When you heat the water. Does Heat Increase Water Pressure.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Does Heat Increase Water Pressure Most solids expand when heated,. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. For example, having water sealed at atmospheric. At higher pressure, the expansion takes more work. When you heat the water it expands, which does work against the surrounding pressure.. Does Heat Increase Water Pressure.
From www.homedit.com
How To Increase Water Pressure In Your Home Does Heat Increase Water Pressure Which means, that much heat is required to. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. For example, having water sealed at atmospheric. Yes, at. Does Heat Increase Water Pressure.
From fizik-fizik.blogspot.com
PHYSICS Form 4 Form5 UNDERSTANDING PRESSURE IN LIQUIDS Does Heat Increase Water Pressure At higher pressure, the expansion takes more work. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Most solids expand when heated,. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Keeping the volume exactly. Does Heat Increase Water Pressure.
From gharpedia.com
Tips on How to Increase Water Pressure in the Shower Does Heat Increase Water Pressure Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. For example, having water sealed at atmospheric. At higher pressure, the expansion takes more work. A large body of water, such as. Latent heat of vapourisation of water at 1 bar,. Does Heat Increase Water Pressure.
From inspectapedia.com
Thermal expansion rate of water Hot Water Expansion Rate & Hot Water Pressure Increase in Hot Does Heat Increase Water Pressure For example, having water sealed at atmospheric. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. A large body of water, such as. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. When you heat. Does Heat Increase Water Pressure.
From teamaustin.com
How to Increase Water Pressure from a Well Austin Plumbing, Heating, Air & Electric Does Heat Increase Water Pressure Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. For example, having water sealed at atmospheric. Most solids expand when heated,. The reason is that water. Does Heat Increase Water Pressure.
From ch301.cm.utexas.edu
heating curve Does Heat Increase Water Pressure A large body of water, such as. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. For example, having water sealed at atmospheric. Most solids expand when heated,. The reason is that water has a greater specific heat than most. Does Heat Increase Water Pressure.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/104 Resource Book Does Heat Increase Water Pressure At higher pressure, the expansion takes more work. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Latent heat of vapourisation of water at 1 bar,. Does Heat Increase Water Pressure.
From www.balkanplumbing.com
How to Increase Water Pressure A Property Owner’s Guide Does Heat Increase Water Pressure Most solids expand when heated,. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Pressure and temperature were fairly well understood in the age of newton. Does Heat Increase Water Pressure.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Does Heat Increase Water Pressure At higher pressure, the expansion takes more work. When you heat the water it expands, which does work against the surrounding pressure. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that. Does Heat Increase Water Pressure.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Yes, at constant density, the pressure increases as the temperature does: A large body of water, such. Does Heat Increase Water Pressure.
From www.wikihow.com
3 Ways to Increase Water Pressure wikiHow Does Heat Increase Water Pressure The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. At higher pressure, the expansion takes more work. Yes, at constant density, the pressure increases as the temperature does: Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Pressure. Does Heat Increase Water Pressure.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Heat Increase Water Pressure Yes, at constant density, the pressure increases as the temperature does: The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. Most solids expand when heated,. For example, having water sealed at atmospheric. At higher pressure, the expansion takes more work. When you heat. Does Heat Increase Water Pressure.
From 4perfectwater.com
How to Increase Water Pressure in Your Home (2020) Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed. Yes, at constant density, the pressure increases as the temperature does: When you heat. Does Heat Increase Water Pressure.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Experiments Does Heat Increase Water Pressure When you heat the water it expands, which does work against the surrounding pressure. Yes, at constant density, the pressure increases as the temperature does: Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. At higher pressure, the expansion takes more work. Keeping the volume exactly constant while increasing the temperature is not as simple. Does Heat Increase Water Pressure.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. Yes, at constant density, the pressure increases as the temperature does: At higher pressure, the expansion takes more work. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. When you heat the water it expands, which does work. Does Heat Increase Water Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. For example, having water sealed at atmospheric. When you heat the water it expands, which does work against the surrounding pressure. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm. Does Heat Increase Water Pressure.
From www.familyhandyman.com
How to Increase Water Pressure in Your House (DIY) Family Handyman Does Heat Increase Water Pressure Most solids expand when heated,. Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. A large body of water, such as. Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. Pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years. Does Heat Increase Water Pressure.
From evelinekhan.blogspot.com
how to increase water pressure in house uk Eveline Khan Does Heat Increase Water Pressure Keeping the volume exactly constant while increasing the temperature is not as simple as it may sound. The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. For example, having water sealed at atmospheric. Most solids expand when heated,. Which means, that much heat. Does Heat Increase Water Pressure.
From www.researchgate.net
PressureTemperature diagram for water. Download Scientific Diagram Does Heat Increase Water Pressure At higher pressure, the expansion takes more work. Yes, at constant density, the pressure increases as the temperature does: Latent heat of vapourisation of water at 1 bar, $100^\circ c$ is $2257 \frac{kj}{kg}$. When you heat the water it expands, which does work against the surrounding pressure. Which means, that much heat is required to. Pressure and temperature were fairly. Does Heat Increase Water Pressure.