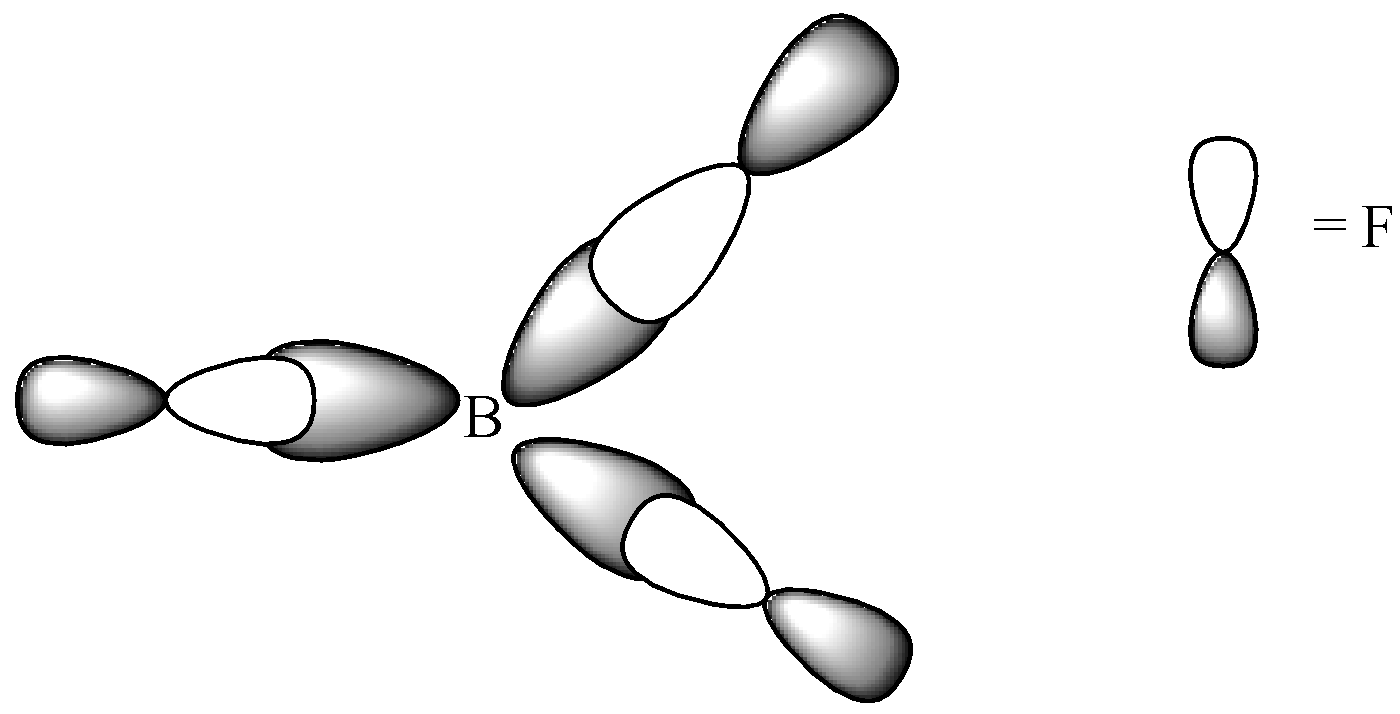
Geometric Shape For Bf3 . The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. The central boron (b) atom in bf3 does not have any lone pairs of electrons. The vsepr notation is ax 3. For a trigonal planar structure, the bond angle is 120°. What is the molecular geometry of bf3? The molecular geometry is the same as the electron geometry. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Click the symmetry operations above to view them in 3d. What is the molecular geometry of bf3? Here’s a breakdown of its structure: What is the bond angle in bf3? The electron geometry of bf 3 is trigonal planar. Bf3 belongs to the d3h point group and. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms.
from www.vedantu.com
Bf3 belongs to the d3h point group and. Click the symmetry operations above to view them in 3d. Here’s a breakdown of its structure: The shape is not distorted because there are no lone pairs on the central boron atom. For a trigonal planar structure, the bond angle is 120°. The central boron (b) atom in bf3 does not have any lone pairs of electrons. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. How many lone pairs are on the central boron (b) atom in bf3? The vsepr notation is ax 3. What is the molecular geometry of bf3?
Explain the formation of the B{{F}_{3}} molecule using hybridisation.
Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Here’s a breakdown of its structure: The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. The electron geometry of bf 3 is trigonal planar. What is the molecular geometry of bf3? An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Bf3 belongs to the d3h point group and. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. What is the bond angle in bf3? The shape is not distorted because there are no lone pairs on the central boron atom. How many lone pairs are on the central boron (b) atom in bf3? The central boron (b) atom in bf3 does not have any lone pairs of electrons. The molecular geometry is the same as the electron geometry. Click the symmetry operations above to view them in 3d. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. Boron trifluoride (bf3) has a trigonal planar molecular geometry.
From www.vedantu.com
Explain the formation of the B{{F}_{3}} molecule using hybridisation. Geometric Shape For Bf3 Click the symmetry operations above to view them in 3d. How many lone pairs are on the central boron (b) atom in bf3? An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Bf3 belongs to the d3h point group and. The shape is not distorted because there are no lone pairs on the central. Geometric Shape For Bf3.
From www.youtube.com
BF3 lewis structure and Molecular Geometry YouTube Geometric Shape For Bf3 Here’s a breakdown of its structure: Bf3 belongs to the d3h point group and. The central boron (b) atom in bf3 does not have any lone pairs of electrons. For a trigonal planar structure, the bond angle is 120°. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three. Geometric Shape For Bf3.
From www.youtube.com
BF3 Boron trifluoride hybridization structure shape bond angle adichemistry chemical bonding Geometric Shape For Bf3 The central boron (b) atom in bf3 does not have any lone pairs of electrons. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. Click the symmetry operations above to view them in 3d. What is the molecular geometry of bf3? The shape is not distorted because there are no lone. Geometric Shape For Bf3.
From www.animalia-life.club
Lewis Dot Structure For Bf3 Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Bf3 belongs to the d3h point group and. Boron trifluoride (bf3) has a trigonal planar molecular geometry. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. What is the molecular geometry of bf3? What is the. Geometric Shape For Bf3.
From karenschroeder.blogspot.com
Label All Bonds In Bf3 / Bf3 Molecular Geometry Science Education And Tutorials Karen Schroeder Geometric Shape For Bf3 The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. Bf3 belongs to the d3h point group and. Click the symmetry operations above to view them in 3d. The vsepr notation is ax 3. Boron trifluoride (bf3) has a trigonal planar molecular geometry. An explanation of the molecular geometry for. Geometric Shape For Bf3.
From www.youtube.com
BF3 Molecular Geometry, Shape and Bond Angles (Boron Trifluoride) YouTube Geometric Shape For Bf3 The shape is not distorted because there are no lone pairs on the central boron atom. What is the molecular geometry of bf3? For a trigonal planar structure, the bond angle is 120°. The central boron (b) atom in bf3 does not have any lone pairs of electrons. Here’s a breakdown of its structure: An explanation of the molecular geometry. Geometric Shape For Bf3.
From www.youtube.com
Hybridization of BF3 (Boron trifluoride) YouTube Geometric Shape For Bf3 The shape is not distorted because there are no lone pairs on the central boron atom. How many lone pairs are on the central boron (b) atom in bf3? Click the symmetry operations above to view them in 3d. The molecular geometry is the same as the electron geometry. What is the bond angle in bf3? What is the molecular. Geometric Shape For Bf3.
From www.youtube.com
4.Symmetry properties of BF3 molecule. YouTube Geometric Shape For Bf3 Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. The molecular geometry is the same as the electron geometry. The vsepr notation is ax 3. What is the molecular geometry of bf3? The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr). Geometric Shape For Bf3.
From www.chegg.com
Solved A model for BF3 is shown in the chem3D window. BF3 Geometric Shape For Bf3 For a trigonal planar structure, the bond angle is 120°. Bf3 belongs to the d3h point group and. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. What is the bond angle in bf3? What is the molecular geometry of bf3? Click the symmetry operations above to view them in 3d.. Geometric Shape For Bf3.
From www.coursehero.com
[Solved] Explain the hybridization and shape of molecule BF3 with hand written Course Hero Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Click the symmetry operations above to view them in 3d. For a trigonal planar structure, the bond angle is 120°. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. The central boron (b) atom. Geometric Shape For Bf3.
From mungfali.com
BF3 Lewis Dot Structure Geometric Shape For Bf3 How many lone pairs are on the central boron (b) atom in bf3? Boron trifluoride (bf3) has a trigonal planar molecular geometry. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. The central boron (b) atom in bf3 does not have. Geometric Shape For Bf3.
From www.youtube.com
BF3 Lewis Structure YouTube Geometric Shape For Bf3 For a trigonal planar structure, the bond angle is 120°. The shape is not distorted because there are no lone pairs on the central boron atom. Boron trifluoride (bf3) has a trigonal planar molecular geometry. What is the molecular geometry of bf3? An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. The electron geometry. Geometric Shape For Bf3.
From www.animalia-life.club
Lewis Dot Structure For Bf3 Geometric Shape For Bf3 Bf3 belongs to the d3h point group and. The electron geometry of bf 3 is trigonal planar. The molecular geometry is the same as the electron geometry. An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. The shape is not distorted because there are no lone pairs on the central boron atom. For a. Geometric Shape For Bf3.
From www.youtube.com
What is the molecular shape of BF3? YouTube Geometric Shape For Bf3 Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. The molecular geometry is the same as the electron geometry. The vsepr notation is ax 3. What is the molecular geometry of bf3? Bf3 belongs to the d3h point group and. The electron geometry of bf 3 is trigonal planar. Boron trifluoride. Geometric Shape For Bf3.
From www.studyxapp.com
a model for bf3 is shown in the chem3d window bf3 has trigonal planar geometry StudyX Geometric Shape For Bf3 The vsepr notation is ax 3. The molecular geometry is the same as the electron geometry. For a trigonal planar structure, the bond angle is 120°. Click the symmetry operations above to view them in 3d. The electron geometry of bf 3 is trigonal planar. The shape is not distorted because there are no lone pairs on the central boron. Geometric Shape For Bf3.
From ellicacing.blogspot.com
Electron Geometry Of Bf3 / Boron Trifluoride BF3 Molecular Geometry & Polarity Draw the lewis Geometric Shape For Bf3 For a trigonal planar structure, the bond angle is 120°. Bf3 belongs to the d3h point group and. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. Click the symmetry operations above to view them in 3d. The electron geometry of bf 3 is trigonal planar. What is the bond angle. Geometric Shape For Bf3.
From www.ck12.org
Molecular Geometry CK12 Foundation Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. What is the bond angle in bf3? For a trigonal planar structure, the bond angle is 120°. Click the symmetry operations above to view them in 3d. How many lone pairs are on the central boron (b) atom in bf3? The central boron (b) atom. Geometric Shape For Bf3.
From sketchfab.com
Geometry BF3_Trigonal planar Download Free 3D model by International Medical University (IMU Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Bf3 belongs to the d3h point group and. How many lone pairs are on the central boron (b) atom in bf3? The molecular geometry is the same as the electron geometry. For a trigonal planar structure, the bond angle is 120°. What is the molecular. Geometric Shape For Bf3.
From techiescientist.com
BF3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist Geometric Shape For Bf3 Bf3 belongs to the d3h point group and. An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. For a trigonal planar structure, the bond angle is 120°. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the. Geometric Shape For Bf3.
From brainly.in
The shape of BF3 molecules is Brainly.in Geometric Shape For Bf3 What is the bond angle in bf3? An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. The vsepr notation is ax 3. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. How many lone pairs are on the central boron (b) atom in. Geometric Shape For Bf3.
From whatsinsight.org
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity What's Insight Geometric Shape For Bf3 The molecular geometry is the same as the electron geometry. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. How many lone pairs are on the central boron (b) atom in bf3? Boron trifluoride. Geometric Shape For Bf3.
From www.youtube.com
BF3 Electron Geometry (Boron trifluoride) YouTube Geometric Shape For Bf3 Click the symmetry operations above to view them in 3d. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. The electron geometry of bf 3 is trigonal planar. The shape is not distorted because there are no lone pairs on the. Geometric Shape For Bf3.
From www.animalia-life.club
Lewis Dot Structure For Bf3 Geometric Shape For Bf3 The electron geometry of bf 3 is trigonal planar. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. Click the symmetry operations above to view them in 3d. For a trigonal planar structure, the bond angle is 120°. The geometry of molecule of bf3 is ‘trigonal planar.’ with the. Geometric Shape For Bf3.
From animalia-life.club
Lewis Dot Structure For Bf3 Geometric Shape For Bf3 The electron geometry of bf 3 is trigonal planar. Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. What is the molecular geometry of bf3? The shape is not distorted because there are no lone pairs on the central boron atom. Bf3 belongs to the d3h point group and. How many. Geometric Shape For Bf3.
From www.chemtube3d.com
Boron Trifluoride (BF3) D3h Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. What is the bond angle in bf3? What is the molecular geometry of bf3? Bf3 belongs to. Geometric Shape For Bf3.
From www.youtube.com
Symmetry elements of BF3Group TheoryDynamic Chemistry YouTube Geometric Shape For Bf3 Bf3 has a trigonal planar molecular geometry, where the central boron atom is surrounded by three fluorine atoms. The shape is not distorted because there are no lone pairs on the central boron atom. The vsepr notation is ax 3. How many lone pairs are on the central boron (b) atom in bf3? The central boron (b) atom in bf3. Geometric Shape For Bf3.
From www.aceorganicchem.com
Molecular Geometry of BF3 [with video and free study guide] Geometric Shape For Bf3 The shape is not distorted because there are no lone pairs on the central boron atom. The central boron (b) atom in bf3 does not have any lone pairs of electrons. Bf3 belongs to the d3h point group and. Click the symmetry operations above to view them in 3d. For a trigonal planar structure, the bond angle is 120°. How. Geometric Shape For Bf3.
From www.youtube.com
Molecular Geometry of BF3 YouTube Geometric Shape For Bf3 The molecular geometry is the same as the electron geometry. The vsepr notation is ax 3. The shape is not distorted because there are no lone pairs on the central boron atom. How many lone pairs are on the central boron (b) atom in bf3? Bf3 belongs to the d3h point group and. Click the symmetry operations above to view. Geometric Shape For Bf3.
From mungfali.com
BF3 VSEPR Shape Geometric Shape For Bf3 What is the bond angle in bf3? The molecular geometry is the same as the electron geometry. For a trigonal planar structure, the bond angle is 120°. What is the molecular geometry of bf3? Boron trifluoride (bf3) has a trigonal planar molecular geometry. The vsepr notation is ax 3. Bf3 belongs to the d3h point group and. Click the symmetry. Geometric Shape For Bf3.
From bilag.xxl.no
Draw The Lewis Structure Of Bf3 Geometric Shape For Bf3 The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. The central boron (b) atom in bf3 does not have any lone pairs of electrons. The vsepr notation is ax 3. For a trigonal planar structure, the bond angle is 120°. An explanation of the molecular geometry for the bf3. Geometric Shape For Bf3.
From www.slideserve.com
PPT What is the shape of BF 3 ? You do not need to take notes, this is just a review on what Geometric Shape For Bf3 Click the symmetry operations above to view them in 3d. The vsepr notation is ax 3. What is the bond angle in bf3? The central boron (b) atom in bf3 does not have any lone pairs of electrons. The shape is not distorted because there are no lone pairs on the central boron atom. The molecular geometry is the same. Geometric Shape For Bf3.
From whatsinsight.org
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity What's Insight Geometric Shape For Bf3 What is the molecular geometry of bf3? Bf3 belongs to the d3h point group and. How many lone pairs are on the central boron (b) atom in bf3? The molecular geometry is the same as the electron geometry. An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Boron trifluoride (bf3) has a trigonal planar. Geometric Shape For Bf3.
From draweasy8.blogspot.com
Bf3 Lewis Structure Electron Geometry Draw Easy Geometric Shape For Bf3 The electron geometry of bf 3 is trigonal planar. Bf3 belongs to the d3h point group and. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. The shape is not distorted because there are no lone pairs on the central boron atom. How many lone pairs are on the. Geometric Shape For Bf3.
From www.slideshare.net
Molecular geometry Geometric Shape For Bf3 An explanation of the molecular geometry for the bf3 (boron trifluoride) including a description of. Boron trifluoride (bf3) has a trigonal planar molecular geometry. Click the symmetry operations above to view them in 3d. The molecular geometry is the same as the electron geometry. What is the bond angle in bf3? The electron geometry of bf 3 is trigonal planar.. Geometric Shape For Bf3.
From topblogtenz.com
BF3 lewis structure, molecular geometry, hybridization, bond angle Geometric Shape For Bf3 Bf3 belongs to the d3h point group and. The vsepr notation is ax 3. The electron geometry of bf 3 is trigonal planar. The molecular shape of bf 3 is trigonal planar, or ax 3 using valence shell electron pair repulsion (vsepr) theory. What is the bond angle in bf3? How many lone pairs are on the central boron (b). Geometric Shape For Bf3.