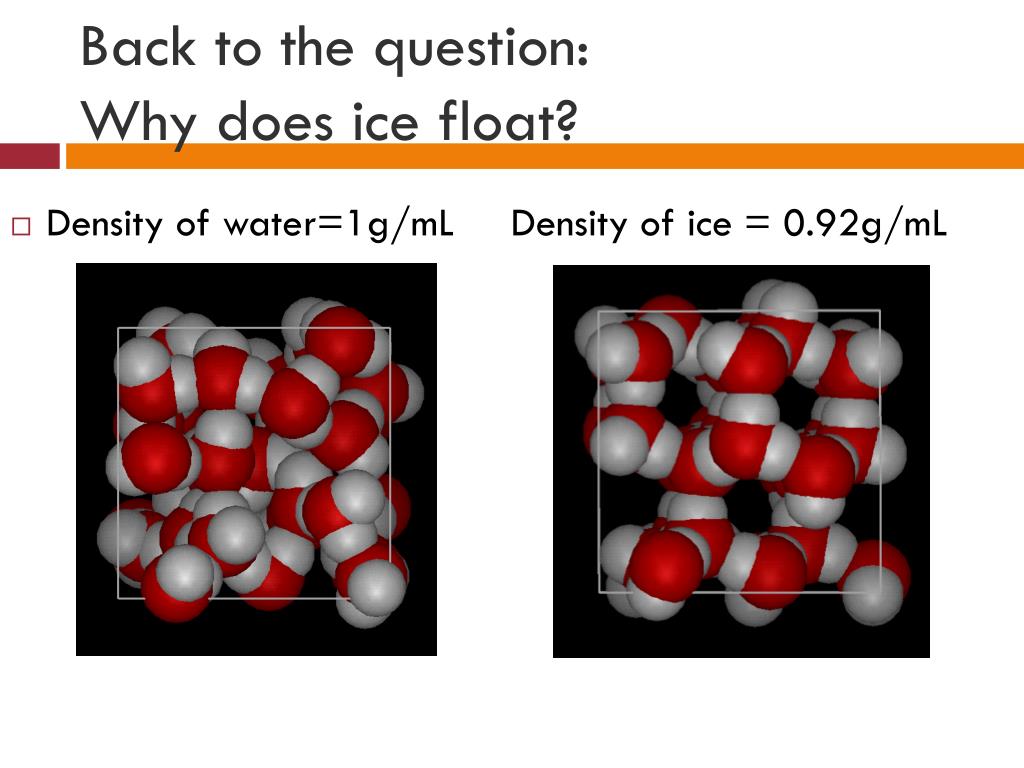
Why Ice Float Density . In other words, ice takes up about 9% more space than water, so a liter of ice. It turns out that ice has a lower. However, this is a peculiar behavior as solid matter. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Water ice, the solid state of water, floats because it is less dense than its liquid form. Ice floats because it is about 9% less dense than liquid water. Most other substances, by contrast, become denser in the solid phase. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. The scientific explanation for why ice floats on water is based on the concept of density.
from www.slideserve.com
At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Water ice, the solid state of water, floats because it is less dense than its liquid form. The scientific explanation for why ice floats on water is based on the concept of density. Most other substances, by contrast, become denser in the solid phase. However, this is a peculiar behavior as solid matter. Ice floats because it is about 9% less dense than liquid water. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. It turns out that ice has a lower. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass.
PPT Polar Bonds and Molecules PowerPoint Presentation, free download
Why Ice Float Density However, this is a peculiar behavior as solid matter. It turns out that ice has a lower. Ice floats because it is about 9% less dense than liquid water. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. In other words, ice takes up about 9% more space than water, so a liter of ice. Most other substances, by contrast, become denser in the solid phase. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. The scientific explanation for why ice floats on water is based on the concept of density. Water ice, the solid state of water, floats because it is less dense than its liquid form. However, this is a peculiar behavior as solid matter. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern.
From loeqwidny.blob.core.windows.net
Why Does Ice Floats On Water Class 9 at Lynda Evans blog Why Ice Float Density Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. However, this is a peculiar behavior as solid matter. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas. Why Ice Float Density.
From www.chegg.com
Solved Which of the following explains why ice floats on Why Ice Float Density The scientific explanation for why ice floats on water is based on the concept of density. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Most other substances, by contrast, become denser in the solid phase. Perhaps a cold, refreshing drink on a hot day wouldn't look so. Why Ice Float Density.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Why Ice Float Density Water ice, the solid state of water, floats because it is less dense than its liquid form. Ice floats because it is about 9% less dense than liquid water. Most other substances, by contrast, become denser in the solid phase. It turns out that ice has a lower. Perhaps a cold, refreshing drink on a hot day wouldn't look so. Why Ice Float Density.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Ice Float Density In other words, ice takes up about 9% more space than water, so a liter of ice. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the. Why Ice Float Density.
From www.slideserve.com
PPT C H A P T E R 11 Fluids PowerPoint Presentation, free download Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. It turns out that ice has a lower. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Ice floats because it is about 9% less dense than liquid water. At zero degrees, i.e., the temperature at which. Why Ice Float Density.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Ice Float Density It turns out that ice has a lower. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. In other words, ice takes up about 9% more space than water, so a liter of ice. Ice floats because it is about 9% less dense than liquid water. However, this. Why Ice Float Density.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Ice Float Density Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice floats because it is about 9% less dense than liquid water. Water. Why Ice Float Density.
From www.youtube.com
Why Ice Floats on Water YouTube Why Ice Float Density In other words, ice takes up about 9% more space than water, so a liter of ice. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to. Why Ice Float Density.
From www.youtube.com
what is Density Why does ice float on water? SI unit, Formula Why Ice Float Density At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Most other substances, by contrast, become denser in the solid phase. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. In other. Why Ice Float Density.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass Why Ice Float Density Water ice, the solid state of water, floats because it is less dense than its liquid form. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to. Why Ice Float Density.
From slideplayer.com
Essential Chemistry for Biology ppt download Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. At. Why Ice Float Density.
From www.slideserve.com
PPT Polar Bonds and Molecules PowerPoint Presentation, free download Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. In other words,. Why Ice Float Density.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the. Why Ice Float Density.
From vervetimes.com
The Reason Why Ice Floats Explained Live Science Verve times Why Ice Float Density However, this is a peculiar behavior as solid matter. In other words, ice takes up about 9% more space than water, so a liter of ice. Ice floats because it is about 9% less dense than liquid water. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. The scientific. Why Ice Float Density.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater and the Why Ice Float Density Ice floats because it is about 9% less dense than liquid water. Most other substances, by contrast, become denser in the solid phase. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. However, this is a peculiar behavior as solid matter. The. Why Ice Float Density.
From www.youtube.com
Density Why Ice floats on Water? YouTube Why Ice Float Density Water ice, the solid state of water, floats because it is less dense than its liquid form. Most other substances, by contrast, become denser in the solid phase. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. However, this is a peculiar behavior as solid matter. It turns. Why Ice Float Density.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater and the Why Ice Float Density Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Water ice, the solid state of water, floats because it is less dense. Why Ice Float Density.
From answerdbmaligner.z21.web.core.windows.net
How Does Ice Float Why Ice Float Density It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. It turns out that ice has a lower. Ice floats because it is about 9% less dense than liquid water. However, this is a peculiar behavior as solid matter. At zero degrees, i.e., the. Why Ice Float Density.
From www.teachoo.com
Liquids generally have lower density as compared to solids. But you Why Ice Float Density However, this is a peculiar behavior as solid matter. In other words, ice takes up about 9% more space than water, so a liter of ice. It turns out that ice has a lower. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. The scientific explanation for why ice. Why Ice Float Density.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas Why Ice Float Density In other words, ice takes up about 9% more space than water, so a liter of ice. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the. Why Ice Float Density.
From slideshare.net
Density Ppt Why Ice Float Density At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if. Why Ice Float Density.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. In other words, ice takes up about 9% more space than water, so a liter of ice. At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice floats because it is about 9% less dense than liquid water.. Why Ice Float Density.
From loeqwidny.blob.core.windows.net
Why Does Ice Floats On Water Class 9 at Lynda Evans blog Why Ice Float Density The scientific explanation for why ice floats on water is based on the concept of density. In other words, ice takes up about 9% more space than water, so a liter of ice. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. Most other substances, by contrast, become. Why Ice Float Density.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas Why Ice Float Density It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Water ice, the solid state of water, floats because it is less dense than its liquid form. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes. Why Ice Float Density.
From www.thoughtco.com
Why Does Ice Float? Ice and the Density of Water Why Ice Float Density Ice floats because it is about 9% less dense than liquid water. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern.. Why Ice Float Density.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. However, this is a peculiar behavior as solid matter. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. It is common for us to observe ice cubes floating when placed in. Why Ice Float Density.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation ID2739092 Why Ice Float Density Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. However, this is a peculiar behavior as solid matter. The scientific explanation for why ice floats on water is based on the concept of density. Ice floats because it is about 9% less dense than liquid water. At zero. Why Ice Float Density.
From dokumen.tips
(PPT) Density, Mass & Volume. Why do you think ice floats in water Why Ice Float Density Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice. The scientific explanation for why ice floats on water is based on the concept of density. Ice is less dense than liquid water because the molecular structure of ice is more. Why Ice Float Density.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC Why Ice Float Density At zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. It turns out that ice has a lower. Most other substances, by contrast, become. Why Ice Float Density.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID9145406 Why Ice Float Density The scientific explanation for why ice floats on water is based on the concept of density. Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice. It is common for us to observe ice cubes floating when placed in a glass. Why Ice Float Density.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Ice Float Density Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. Water ice, the solid state of water, floats because it is less dense than its liquid form. In other words, ice takes up about 9% more space than water, so a liter of. Why Ice Float Density.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii Why Ice Float Density Most other substances, by contrast, become denser in the solid phase. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. In other words, ice takes up about 9% more space than water, so a liter of ice. It turns out that ice has a lower. The scientific explanation. Why Ice Float Density.
From www.slideserve.com
PPT Aim How do we calculate density? PowerPoint Presentation, free Why Ice Float Density It turns out that ice has a lower. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. Water ice, the solid state of water, floats because it is less dense than its liquid form. Ice is less dense than liquid water because. Why Ice Float Density.
From www.youtube.com
Why Ice floats on water... basic concept.. ice density Why Ice Float Density In other words, ice takes up about 9% more space than water, so a liter of ice. However, this is a peculiar behavior as solid matter. Ice is less dense than liquid water because the molecular structure of ice is more spread out, forming a hexagonal pattern. It turns out that ice has a lower. Most other substances, by contrast,. Why Ice Float Density.
From www.zmescience.com
The reason why ice floats Why Ice Float Density In other words, ice takes up about 9% more space than water, so a liter of ice. Ice floats because it is about 9% less dense than liquid water. Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. At zero degrees, i.e.,. Why Ice Float Density.