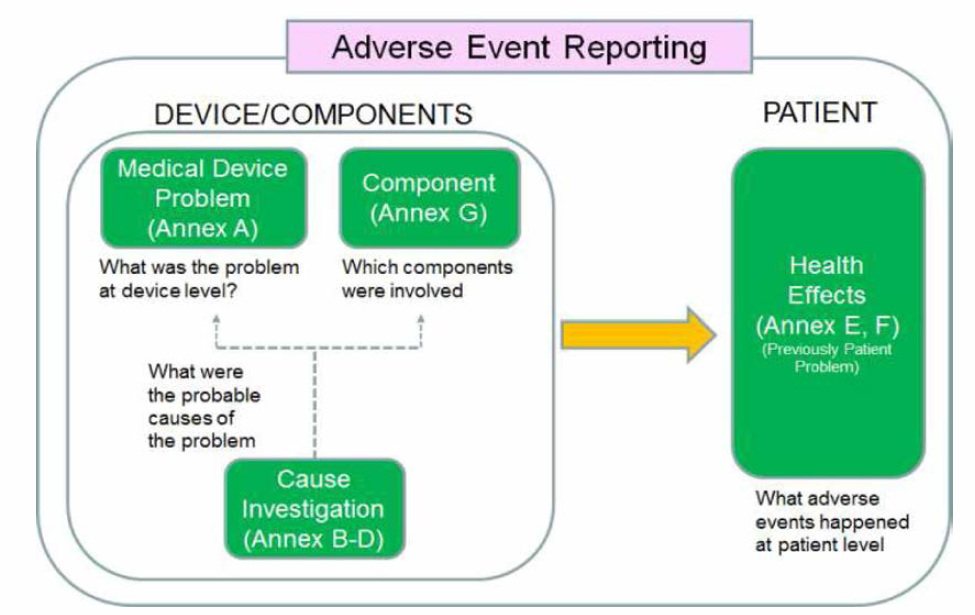
Imdrf Medical Device Problem Codes (Annex E) . It is recognized that not all jurisdictions may want to code to. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex a provides a comprehensive list of medical device problem terms and codes. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices.
from giojpmkll.blob.core.windows.net
Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex a provides a comprehensive list of medical device problem terms and codes. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. It is recognized that not all jurisdictions may want to code to.
Imdrf Medical Device Problem Codes (Annex A) at Anna Tyler blog
Imdrf Medical Device Problem Codes (Annex E) Annex a provides a comprehensive list of medical device problem terms and codes. It is recognized that not all jurisdictions may want to code to. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices.
From www.greenlight.guru
Top 40 IMDRF Technical Documents for Medical Devices Imdrf Medical Device Problem Codes (Annex E) 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex a provides a comprehensive list of medical device problem terms and codes. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. It is recognized that. Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
Adverse Event Terminology of IMDRF Annex F Codes Means Health Impact Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. The fda is a participant in the imdrf adverse event terminology working group, which aims to. Imdrf Medical Device Problem Codes (Annex E).
From www.johner-institut.de
IMDRF, das "International Medical Device Regulators Forum" Imdrf Medical Device Problem Codes (Annex E) Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Annex a provides a comprehensive list of. Imdrf Medical Device Problem Codes (Annex E).
From www.pdffiller.com
Fillable Online IMDRF Presentation Integrating Device Registries UDI Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to. Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
IMDRF Guidance on Application of Risk Management Principles to Design Imdrf Medical Device Problem Codes (Annex E) It is recognized that not all jurisdictions may want to code to. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex a provides a comprehensive list of medical device problem terms and codes. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize.. Imdrf Medical Device Problem Codes (Annex E).
From qbdgroup.com
The regulatory pathway for your custommade medical device QbD Group Imdrf Medical Device Problem Codes (Annex E) Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. It is recognized that not all jurisdictions may want. Imdrf Medical Device Problem Codes (Annex E).
From array.aami.org
Documenting Medical Device Risk Management through the Risk Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Imdrf provides standardized terms,. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
MD Guidance, Annex 2, IMDRF Standards Checklist Modified by EU in Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex a provides a comprehensive list of medical device problem terms and codes. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Clinical signs, symptoms. Imdrf Medical Device Problem Codes (Annex E).
From www.vde.com
IMDRFCodes Meaning and Application for Medical Devices Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Imdrf provides standardized terms, terminology and codes for reporting adverse events related. Imdrf Medical Device Problem Codes (Annex E).
From pdfslide.net
(PPT) Software as a Medical Device (SaMD) Application of Quality Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the. Imdrf Medical Device Problem Codes (Annex E).
From medenvoyglobal.com
Standardizing Adverse Event Reporting IMDRF Terminology Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: The fda is. Imdrf Medical Device Problem Codes (Annex E).
From www.aligned.ch
The IMDRF terminologies a common risk language Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex a provides a comprehensive list of medical device problem terms and codes. It is recognized. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
Imdrf PDF Medical Device Medical Diagnosis Imdrf Medical Device Problem Codes (Annex E) 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Clinical signs, symptoms and conditions. Imdrf Medical Device Problem Codes (Annex E).
From giojpmkll.blob.core.windows.net
Imdrf Medical Device Problem Codes (Annex A) at Anna Tyler blog Imdrf Medical Device Problem Codes (Annex E) Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex a provides a comprehensive list of medical device problem terms and codes. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. The fda is a participant in the. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
IMDRF GRRP WG N52 (Edition 2) Principles of Labelling For Medical Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): It is recognized that. Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
Example of IMDRF's SaMDs Risk Categorization Interfacing with Imdrf Medical Device Problem Codes (Annex E) 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex a provides a comprehensive list of medical device problem terms and codes. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex e is a. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
IMDRF NCAR WG N14Final 2022 PDF Medical Device Surgery Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: It is recognized that not all jurisdictions may want to code to. Annex. Imdrf Medical Device Problem Codes (Annex E).
From mdlaw.eu
MDCG on EMDN & IMDRF (UDI) · MDlaw Information platform on European Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Annex a provides a comprehensive list of medical device problem terms and codes. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse. Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
Adverse Event Terminology of IMDRF Annex F Codes Means Health Impact Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological. Imdrf Medical Device Problem Codes (Annex E).
From omcmedical.com
7 IMDRF Regulation on SaMD OMC Medical Limited Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex a provides a comprehensive list of medical device problem terms and codes. It is recognized that not all jurisdictions may want to code to. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Clinical signs, symptoms and conditions (3 levels). Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
(PDF) Risk Management of AI/ML Software as a Medical Device (SaMD) On Imdrf Medical Device Problem Codes (Annex E) Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Imdrf provides standardized. Imdrf Medical Device Problem Codes (Annex E).
From www.researchgate.net
Adverse Event Terminology of IMDRF Annex F Codes Means Health Impact Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f:. Imdrf Medical Device Problem Codes (Annex E).
From medicalmountains.de
Gemeinschaftliche Übersetzung IMDRFCodes MedicalMountains Tuttlingen Imdrf Medical Device Problem Codes (Annex E) Annex a provides a comprehensive list of medical device problem terms and codes. It is recognized that not all jurisdictions may want to code to. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex e is a hierarchical list of terms for reporting adverse. Imdrf Medical Device Problem Codes (Annex E).
From dokumen.tips
(DOCX) Assembly and Technical Guide for IMDRF Table of Contents Imdrf Medical Device Problem Codes (Annex E) 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): It is recognized that not all jurisdictions may want to code to. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and conditions (3 levels) (structured according to. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
Imdrf Proc 141121 Information Standards PDF Medical Device Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Imdrf provides standardized terms, terminology and codes for reporting. Imdrf Medical Device Problem Codes (Annex E).
From www.greenlight.guru
SaMD Software as a Medical Device [The Ultimate Guide] Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: The fda is a participant in the imdrf adverse event terminology working group, which aims to. Imdrf Medical Device Problem Codes (Annex E).
From www.scribd.com
IMDRF 20190321Principles of Labelling PDF Medical Device Barcode Imdrf Medical Device Problem Codes (Annex E) Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Annex a provides a comprehensive list of medical. Imdrf Medical Device Problem Codes (Annex E).
From studylib.net
IMDRF Document Template International Medical Device Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex a provides a comprehensive list of medical device problem. Imdrf Medical Device Problem Codes (Annex E).
From www.johner-institut.de
IMDRF, das "International Medical Device Regulators Forum" Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. Annex a provides a comprehensive. Imdrf Medical Device Problem Codes (Annex E).
From www.makrocare.com
EUDAMED and EU Medical Device Nomenclature MakroCare Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes. Clinical signs, symptoms and. Imdrf Medical Device Problem Codes (Annex E).
From cybellum.com
Principles and Practices for Medical Device Cybersecurity IMDRF/CYBER Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex a provides a comprehensive list of medical device problem terms and codes. It is recognized that not all jurisdictions may want to code to. Imdrf provides standardized terms, terminology and codes. Imdrf Medical Device Problem Codes (Annex E).
From www.greenlight.guru
SaMD Software as a Medical Device [The Ultimate Guide] Imdrf Medical Device Problem Codes (Annex E) The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex e is a hierarchical list of terms for reporting adverse events related to medical devices.. Imdrf Medical Device Problem Codes (Annex E).
From formiventos.com
Medical Device Regulatory Review Report Guidance Regarding Information Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: The fda is a participant in the imdrf adverse event terminology working group, which aims to improve and harmonize. Annex a provides a comprehensive list of medical device problem. Imdrf Medical Device Problem Codes (Annex E).
From www.heartlungcirc.org
Regulatory Requirements For Medical Devices And Vascular Ageing An Imdrf Medical Device Problem Codes (Annex E) Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. It is recognized that not all jurisdictions may want to code to. 療機器規制当局フォーラム(imdrf)にて取りまとめられた「imdrf terminolog ies for categorized adverse event reporting (aer): Annex a provides a comprehensive list of medical device problem terms and codes. The fda is a participant in the imdrf adverse event terminology. Imdrf Medical Device Problem Codes (Annex E).
From matrixreq.com
IMDRF published a new guidance on SBOM for medical device cybersecurity Imdrf Medical Device Problem Codes (Annex E) Clinical signs, symptoms and conditions (3 levels) (structured according to organ / physiological system) annex f: Annex e is a hierarchical list of terms for reporting adverse events related to medical devices. Imdrf provides standardized terms, terminology and codes for reporting adverse events related to medical devices. Annex a provides a comprehensive list of medical device problem terms and codes.. Imdrf Medical Device Problem Codes (Annex E).