Battery Electrochemical Potentials . Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical.
from www.chegg.com
This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Consider a proton exchange membrane (pem).
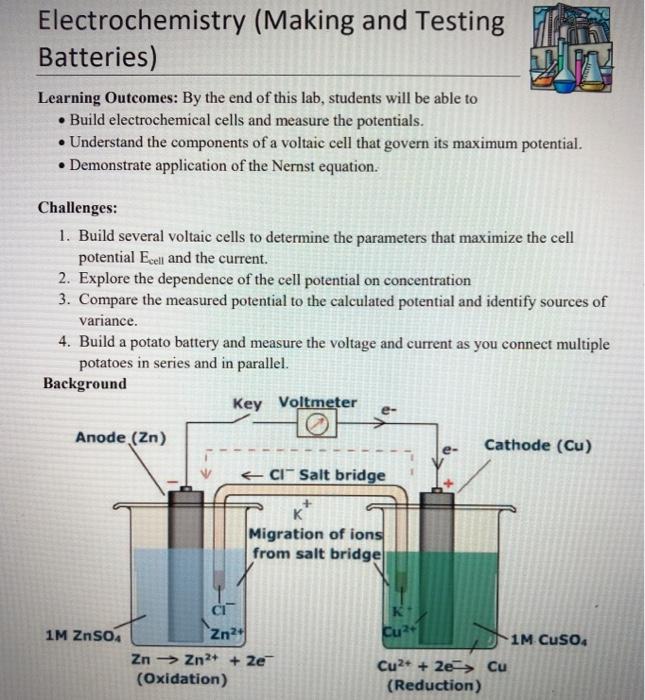
Solved Electrochemistry (Making and Testing Team Batteries)
Battery Electrochemical Potentials Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Consider a proton exchange membrane (pem). This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage.
From www.chegg.com
Solved List the electrochemical series you developed from Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Consider. Battery Electrochemical Potentials.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Battery Electrochemical Potentials Consider a proton exchange membrane (pem). Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different. Battery Electrochemical Potentials.
From 2012books.lardbucket.org
Electrochemistry Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The. Battery Electrochemical Potentials.
From www.pinterest.com
Understanding purpose of salt bridge Galvanic cell, Electrochemical Battery Electrochemical Potentials The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the. Battery Electrochemical Potentials.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion. Battery Electrochemical Potentials.
From www.thoughtco.com
How to Define Anode and Cathode Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement.. Battery Electrochemical Potentials.
From pressbooks.bccampus.ca
5.3 Galvanic Cells Chemistry for Chemical Engineers Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Consider. Battery Electrochemical Potentials.
From giodwrxvk.blob.core.windows.net
Electrodes In Electrolysis at Lindsay Macy blog Battery Electrochemical Potentials This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction. Battery Electrochemical Potentials.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Battery Electrochemical Potentials Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the. Battery Electrochemical Potentials.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry Battery Electrochemical Potentials This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Consider. Battery Electrochemical Potentials.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy Battery Electrochemical Potentials Consider a proton exchange membrane (pem). This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. The energy density of a rechargeable battery is determined collectively by the specific. Battery Electrochemical Potentials.
From in.pinterest.com
Electrochemistry Ectrochemical Series Electrochemistry, Chemistry Battery Electrochemical Potentials Consider a proton exchange membrane (pem). This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the. Battery Electrochemical Potentials.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Battery Electrochemical Potentials The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Battery Electrochemical Potentials.
From www.science.org
Permeable voidfree interface for allsolidstate alkaliion polymer Battery Electrochemical Potentials Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Consider a proton exchange membrane (pem). The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. The energy density of a rechargeable battery is determined collectively by the. Battery Electrochemical Potentials.
From saylordotorg.github.io
Standard Potentials Battery Electrochemical Potentials Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Consider a proton exchange membrane (pem). The energy density of a rechargeable battery is determined collectively by the specific capacity. Battery Electrochemical Potentials.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Battery Electrochemical Potentials The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed. Battery Electrochemical Potentials.
From batteryindustry.tech
COMSOL battery modeling and simulation provide an efficient and low Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Consider a proton exchange membrane (pem). The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed. Battery Electrochemical Potentials.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Voltaic Cell Battery Electrochemical Potentials This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. The energy density of a rechargeable battery is determined collectively by the specific. Battery Electrochemical Potentials.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Battery Electrochemical Potentials Consider a proton exchange membrane (pem). Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different. Battery Electrochemical Potentials.
From www.pinterest.com
Kognity Chemistry classroom, Electrochemistry, Chemistry lessons Battery Electrochemical Potentials The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,.. Battery Electrochemical Potentials.
From learningcampusstall.z21.web.core.windows.net
Electrochemical Cells A Level Chemistry Battery Electrochemical Potentials Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Consider a proton exchange membrane (pem). The energy density of a rechargeable battery is determined collectively by the specific. Battery Electrochemical Potentials.
From dokumen.tips
(PDF) Understanding electrochemical potentials of cathode...of Li Battery Electrochemical Potentials Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage.. Battery Electrochemical Potentials.
From www.chegg.com
Solved Electrochemistry (Making and Testing Team Batteries) Battery Electrochemical Potentials The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. The energy density of a rechargeable battery is determined collectively by the. Battery Electrochemical Potentials.
From chem.libretexts.org
5.4 Day 39 Voltaic Cells, HalfCell Potentials Chemistry LibreTexts Battery Electrochemical Potentials The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,.. Battery Electrochemical Potentials.
From 2012books.lardbucket.org
Describing Electrochemical Cells Battery Electrochemical Potentials Consider a proton exchange membrane (pem). Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed discussion of potential, galvanic, and impedance measurement. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. The energy density of a rechargeable battery is determined collectively by the specific capacity. Battery Electrochemical Potentials.
From pandai.me
Standard Electrode Potential Battery Electrochemical Potentials The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Consider a proton exchange membrane (pem). This chapter presents an overview of the key concepts, a brief history of the advancement. Battery Electrochemical Potentials.
From saylordotorg.github.io
Electrochemistry Battery Electrochemical Potentials This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. Consider a proton exchange membrane (pem). The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed. Battery Electrochemical Potentials.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Battery Electrochemical Potentials Consider a proton exchange membrane (pem). The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction and. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction potentials,. Starting from an introduction of the basic electrochemistry concepts, the authors offer a detailed. Battery Electrochemical Potentials.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Battery Electrochemical Potentials This chapter presents an overview of the key concepts, a brief history of the advancement and factors governing the electrochemical. The energy density of a rechargeable battery is determined collectively by the specific capacity of electrodes and the working voltage. The voltage or potential difference between an oxidation and reduction reaction arises from the different electrochemical potentials of the reduction. Battery Electrochemical Potentials.