Medical Device Sop Examples . Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. If you are a medical device company, the fda requires you to not. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Sops are the backbone of quality in the medical device industry. Explore the key considerations for standard operating procedures (sops) for medical device companies. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Your emphasis on clarity, consistency, and compliance resonates well. Here are some common examples of when an sop might be solicited for your device:
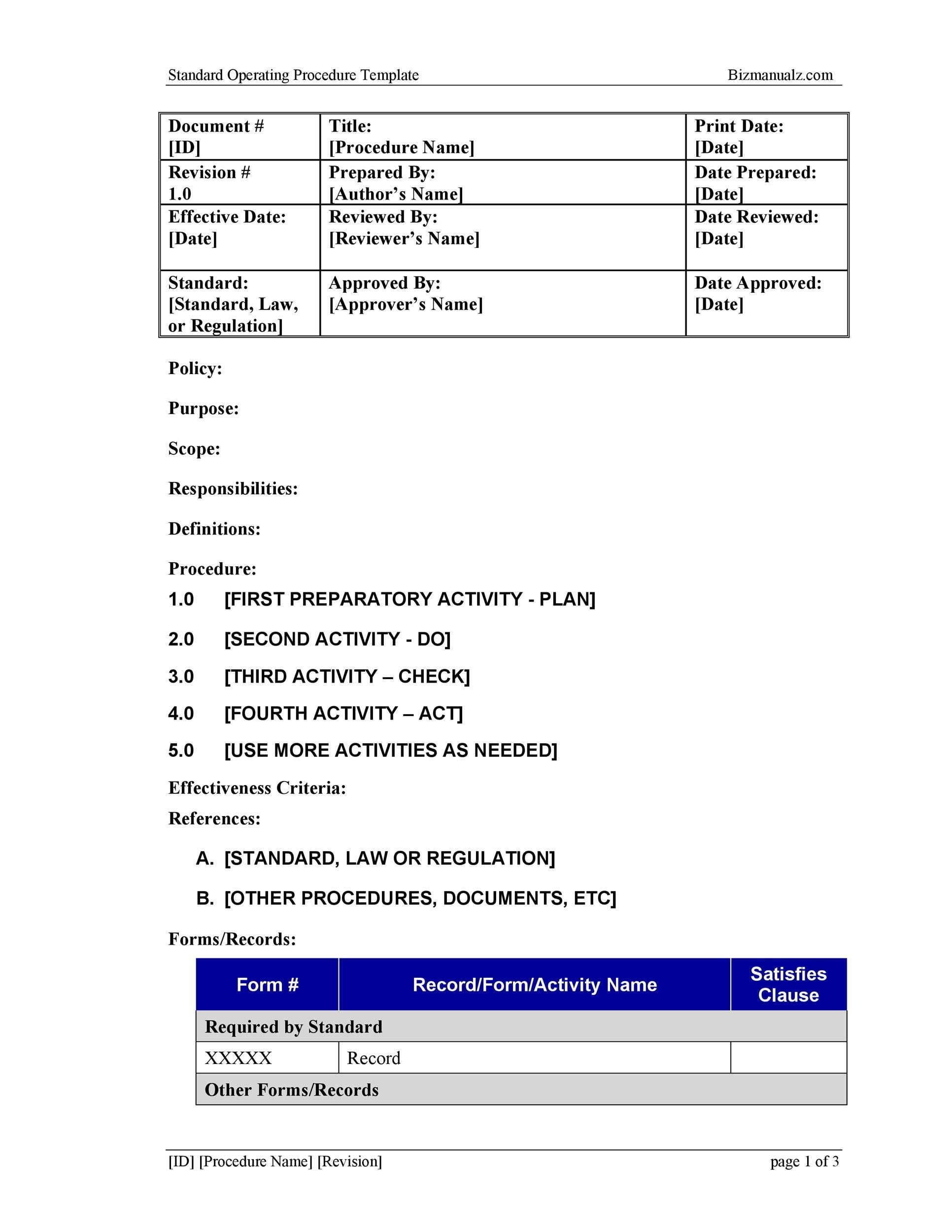
from templatelab.com
Your emphasis on clarity, consistency, and compliance resonates well. Sops are the backbone of quality in the medical device industry. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. If you are a medical device company, the fda requires you to not. Explore the key considerations for standard operating procedures (sops) for medical device companies. Here are some common examples of when an sop might be solicited for your device:
37 Best Standard Operating Procedure (SOP) Templates
Medical Device Sop Examples Your emphasis on clarity, consistency, and compliance resonates well. Here are some common examples of when an sop might be solicited for your device: Your emphasis on clarity, consistency, and compliance resonates well. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Sops are the backbone of quality in the medical device industry. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. If you are a medical device company, the fda requires you to not. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Explore the key considerations for standard operating procedures (sops) for medical device companies.
From gionxxche.blob.core.windows.net
Medical Device Standard That Concerns Clinical Trials at Ernest Best blog Medical Device Sop Examples Explore the key considerations for standard operating procedures (sops) for medical device companies. If you are a medical device company, the fda requires you to not. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. 1) document control, 2) manufacturing processes, 3) transporting devices,. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Sops are the backbone of. Medical Device Sop Examples.
From www.vrogue.co
Sop Templates Medical Device Materials Control vrogue.co Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Here are some common examples of when an sop might be solicited for your device: 1) document control, 2) manufacturing. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples Explore the key considerations for standard operating procedures (sops) for medical device companies. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Your emphasis on clarity, consistency, and compliance resonates well. Sops are the. Medical Device Sop Examples.
From pharmadocx.com
software as a medical device classes Archives Pharmadocx Consultants Medical Device Sop Examples If you are a medical device company, the fda requires you to not. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Sops are the backbone of quality in the medical device industry. Your emphasis on clarity, consistency, and compliance resonates well. This starfish eguide covers 7 steps to. Medical Device Sop Examples.
From www.csensems.com
What is Standard Operating Procedure (SOP)? How to create it effectively? Medical Device Sop Examples If you are a medical device company, the fda requires you to not. Sops are the backbone of quality in the medical device industry. Explore the key considerations for standard operating procedures (sops) for medical device companies. Your emphasis on clarity, consistency, and compliance resonates well. This article covers how to create controlling documents that are essential for a qms. Medical Device Sop Examples.
From blog.sierralabs.com
How to Create Successful SOPs for your Medical Device Medical Device Sop Examples Here are some common examples of when an sop might be solicited for your device: Explore the key considerations for standard operating procedures (sops) for medical device companies. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Writing and. Medical Device Sop Examples.
From www.aplyon.com
Medical Device Product Performance Specification Procedure Medical Device Sop Examples Sops are the backbone of quality in the medical device industry. Here are some common examples of when an sop might be solicited for your device: Explore the key considerations for standard operating procedures (sops) for medical device companies. Your emphasis on clarity, consistency, and compliance resonates well. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). If you. Medical Device Sop Examples.
From operonstrategist.com
Amazing Ways To Write Efficient SOPs For Medical Devices Medical Device Sop Examples If you are a medical device company, the fda requires you to not. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Explore the key considerations for standard operating procedures (sops) for medical device companies. This article covers how to create controlling documents that are essential for a qms. Medical Device Sop Examples.
From template.mapadapalavra.ba.gov.br
Pharmacy Sop Template Medical Device Sop Examples If you are a medical device company, the fda requires you to not. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Your emphasis on clarity, consistency, and compliance resonates well. Learn what sops are, when they are required, and how to write them clearly and concisely for medical. Medical Device Sop Examples.
From old.sermitsiaq.ag
Medical Device Sop Templates Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Sops are the backbone of quality in the medical device industry. Learn what sops are, when they are required, and how to write them clearly and. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples Your emphasis on clarity, consistency, and compliance resonates well. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). If you are a medical device company, the fda requires you to not. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. This article covers how to create controlling documents. Medical Device Sop Examples.
From www.jiosaavn.com
Experience VNS At Home/Work With This Portable Vagus Nerve Stimulation Medical Device Sop Examples Explore the key considerations for standard operating procedures (sops) for medical device companies. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Your emphasis on clarity, consistency, and compliance resonates well. This article covers how to create controlling documents that are essential for a qms with a focus on. Medical Device Sop Examples.
From templates.rjuuc.edu.np
Human Resources Sop Template Medical Device Sop Examples 1) document control, 2) manufacturing processes, 3) transporting devices, 4). If you are a medical device company, the fda requires you to not. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. This starfish eguide covers 7 steps to write an effective medical device. Medical Device Sop Examples.
From westernmotodrags.com
Sop Example Business Mentor Medical Device Sop Examples 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Here are some common examples of when an sop might be solicited for your device: This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Explore the key considerations for standard operating procedures (sops) for medical device companies. Your emphasis on clarity, consistency, and compliance resonates. Medical Device Sop Examples.
From www.qmdocs.com
Medical Device Design Control SOP Medical Device Sop Examples Your emphasis on clarity, consistency, and compliance resonates well. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Sops are the backbone of quality in the medical device industry. Here are some common examples of when an sop might. Medical Device Sop Examples.
From gionxxche.blob.core.windows.net
Medical Device Standard That Concerns Clinical Trials at Ernest Best blog Medical Device Sop Examples Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Sops are the backbone of quality in the medical device industry. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Your emphasis on clarity, consistency, and compliance resonates well. Writing and implementing standard operating procedures (sops) for the manufacture. Medical Device Sop Examples.
From issuu.com
md001medicaldevicesdesigncontrolsop1.022 by qmdocs qmdocs Issuu Medical Device Sop Examples Your emphasis on clarity, consistency, and compliance resonates well. Sops are the backbone of quality in the medical device industry. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies.. Medical Device Sop Examples.
From pkjcourseworkngb.web.fc2.com
How to write an sop template Medical Device Sop Examples Here are some common examples of when an sop might be solicited for your device: Your emphasis on clarity, consistency, and compliance resonates well. Sops are the backbone of quality in the medical device industry. Explore the key considerations for standard operating procedures (sops) for medical device companies. This article covers how to create controlling documents that are essential for. Medical Device Sop Examples.
From giojpqdxl.blob.core.windows.net
Hot Air Oven Pdf In Hindi at Roger Figaro blog Medical Device Sop Examples This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Explore the key considerations for standard operating procedures (sops) for medical device companies. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Learn what sops are, when they are required, and how to write. Medical Device Sop Examples.
From www.linkedin.com
Yuma Konishi, Ph.D. 小西 雄馬 on LinkedIn polymer cdm cdmo penang Medical Device Sop Examples This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Your emphasis on clarity, consistency, and compliance resonates well. If you are a medical device company, the fda requires you to not. Here are some common. Medical Device Sop Examples.
From www.scribd.com
Medical Device Design Verification SOP Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Here are some common examples of when an sop might be solicited for your device: Your emphasis on clarity, consistency, and compliance resonates well. Explore the key considerations for standard operating procedures (sops) for medical device companies. Learn what sops. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Your emphasis on clarity, consistency, and compliance resonates well. If you are a medical device company, the fda requires you to not. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Writing and implementing. Medical Device Sop Examples.
From www.sampletemplates.com
FREE 61+ SOP Templates in PDF MS Word Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. If you are a medical device company, the fda requires you to not. Here are some common examples of when an sop might be solicited for your device: Sops are the backbone of quality in the medical device industry. Explore. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples Your emphasis on clarity, consistency, and compliance resonates well. If you are a medical device company, the fda requires you to not. Explore the key considerations for standard operating procedures (sops) for medical device companies. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents.. Medical Device Sop Examples.
From www.qmdocs.com
Medical Device Design Control SOP Medical Device Sop Examples Explore the key considerations for standard operating procedures (sops) for medical device companies. Sops are the backbone of quality in the medical device industry. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Here are some common examples of when an sop might be solicited for your device: This. Medical Device Sop Examples.
From www.scribd.com
Medical Devices SOP 6 Service Repair and Maintenance of Medical Medical Device Sop Examples This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. 1) document control, 2) manufacturing processes,. Medical Device Sop Examples.
From digitalismedical.com
Standard Operating Procedures In Healthcare Digitalis Medical Medical Device Sop Examples This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. Explore the key considerations for standard operating procedures (sops) for medical device companies. 1) document. Medical Device Sop Examples.
From gionxxche.blob.core.windows.net
Medical Device Standard That Concerns Clinical Trials at Ernest Best blog Medical Device Sop Examples Here are some common examples of when an sop might be solicited for your device: Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. If you are a medical device company, the fda requires you. Medical Device Sop Examples.
From es.scribd.com
Laboratory Management SOP Sample Personal Protective Equipment Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. Your emphasis on clarity, consistency, and compliance resonates well. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Sops are the backbone of quality in the medical device industry. If you are a medical device company, the fda requires. Medical Device Sop Examples.
From www.spierssafety.co.uk
A Guide to SOPs Medical Device Sop Examples This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Your emphasis on clarity, consistency, and compliance resonates well. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Learn what sops are, when they are required, and how to write. Medical Device Sop Examples.
From www.greenlight.guru
How to Write Effective SOPs for Medical Devices Medical Device Sop Examples Explore the key considerations for standard operating procedures (sops) for medical device companies. Here are some common examples of when an sop might be solicited for your device: This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Your emphasis on clarity, consistency, and compliance resonates well. 1) document control, 2) manufacturing processes, 3) transporting devices,. Medical Device Sop Examples.
From gionxxche.blob.core.windows.net
Medical Device Standard That Concerns Clinical Trials at Ernest Best blog Medical Device Sop Examples Here are some common examples of when an sop might be solicited for your device: Sops are the backbone of quality in the medical device industry. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Your emphasis on clarity, consistency, and compliance resonates well. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Learn. Medical Device Sop Examples.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Medical Device Sop Examples Sops are the backbone of quality in the medical device industry. This starfish eguide covers 7 steps to write an effective medical device manufacturing sop. Learn what sops are, when they are required, and how to write them clearly and concisely for medical device companies. 1) document control, 2) manufacturing processes, 3) transporting devices, 4). Your emphasis on clarity, consistency,. Medical Device Sop Examples.
From adswan.com
Medical Device SOP Templates Download SINGAPORE FREE Medical Device Sop Examples Writing and implementing standard operating procedures (sops) for the manufacture of medical devices is required by iso 13485, fda, and. This article covers how to create controlling documents that are essential for a qms with a focus on the purpose and scope of the documents. Your emphasis on clarity, consistency, and compliance resonates well. This starfish eguide covers 7 steps. Medical Device Sop Examples.