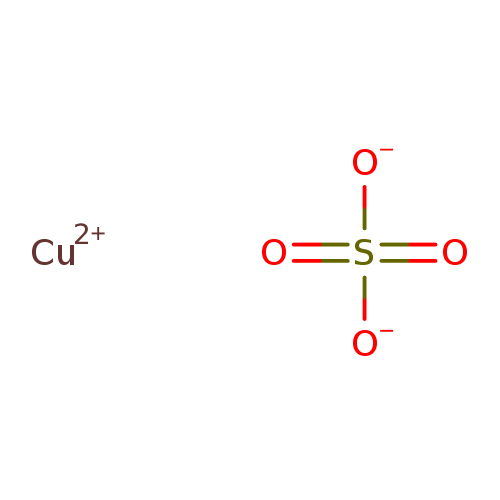
Copper Ii Sulfate Acid Or Base . if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). Note, hydrogen can not lose its only electron as then it. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. It can react with bases to. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone.
from www.t3db.ca
to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. It can react with bases to. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). Note, hydrogen can not lose its only electron as then it. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone.
T3DB Copper(II) sulfate
Copper Ii Sulfate Acid Or Base if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). Note, hydrogen can not lose its only electron as then it. It can react with bases to. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound.
From www.flinnsci.com
Copper(II) Sulfate, Powder, Lab Grade, 500 g Flinn Scientific Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). copper (ii) sulfate,. Copper Ii Sulfate Acid Or Base.
From exotjinqn.blob.core.windows.net
Copper Ii Sulfate Chemical Formula at Jack Butler blog Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). It can react with bases to. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. to tell if cuso4 (copper. Copper Ii Sulfate Acid Or Base.
From www.t3db.ca
T3DB Copper(II) sulfate Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. Note, hydrogen can not lose its only electron as then it. It can react with bases to. in aqueous solutions, copper(ii) sulfate. Copper Ii Sulfate Acid Or Base.
From www.youtube.com
Preparation & Properties of Ammonium Copper (II) sulfate YouTube Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. It can react with bases to. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due. Copper Ii Sulfate Acid Or Base.
From www.copper-powder99.com
Chemical Formula For Copper Sulfate Yosoar Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of. Copper Ii Sulfate Acid Or Base.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper,. Copper Ii Sulfate Acid Or Base.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. It can react with bases to. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Hydrated copper (II) sulphate Stock Image C009/9431 Science Photo Library Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. Note, hydrogen can not lose its only electron as. Copper Ii Sulfate Acid Or Base.
From www.dreamstime.com
Copper(II) Sulfate with Molecular Structure. Chemical Ingredient Used in Medical and Public Copper Ii Sulfate Acid Or Base the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. It can react with bases to. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. the standard answer is yes, because hx2sox4 h x 2 s o. Copper Ii Sulfate Acid Or Base.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Acid Or Base the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. It can react with bases to. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic. Copper Ii Sulfate Acid Or Base.
From www.alamy.com
Copper(II) sulfate with chemical formula. Chemical ingredient used in medical and public health Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). Note, hydrogen can not lose its only electron as then it. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. the systematic name for. Copper Ii Sulfate Acid Or Base.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used in medical and public Copper Ii Sulfate Acid Or Base It can react with bases to. Note, hydrogen can not lose its only electron as then it. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. if it is an acid, we base it's name on the ionic. Copper Ii Sulfate Acid Or Base.
From exospwven.blob.core.windows.net
Copper (Ii) Sulfate Is The And Water Is The at Ben Pratt blog Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. It can react with bases to. to tell if cuso4 (copper (ii) sulfate) forms an. Copper Ii Sulfate Acid Or Base.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper Ii Sulfate Acid Or Base It can react with bases to. Note, hydrogen can not lose its only electron as then it. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. the systematic name for cuso 4 is copper(ii) sulfate, but it is. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Copper II sulphate pentahydrate Stock Image C028/4192 Science Photo Library Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. Note, hydrogen can not lose its only electron as. Copper Ii Sulfate Acid Or Base.
From www.carolina.com
Copper (II) Sulfate, Anhydrous, Reagent Grade, 100 g Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. It can react with bases to. the. Copper Ii Sulfate Acid Or Base.
From www.youtube.com
DEMONSTRATING USING COPPER(II) SULFATE TO DETECT WATER A REVERSIBLE REACTION (GCSE CHEMISTRY Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the standard answer. Copper Ii Sulfate Acid Or Base.
From www.pngwing.com
Copper(II) sulfate Crystal structure Chemical compound, salt, natural Foods, food, chemistry png Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4. Copper Ii Sulfate Acid Or Base.
From exotjinqn.blob.core.windows.net
Copper Ii Sulfate Chemical Formula at Jack Butler blog Copper Ii Sulfate Acid Or Base It can react with bases to. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic. Copper Ii Sulfate Acid Or Base.
From www.flinnsci.com
Copper(II) Sulfate, Powder, Lab Grade, 2 kg Flinn Scientific Copper Ii Sulfate Acid Or Base the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. Note, hydrogen can not lose its only electron as then it. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also. Copper Ii Sulfate Acid Or Base.
From www.amazon.com
Copper (II) Sulfate Solution, 1M, 500mL The Curated Chemical Collection Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. It can react with bases to. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula. Copper Ii Sulfate Acid Or Base.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Note, hydrogen can not lose its only electron as then it. if it is an acid, we. Copper Ii Sulfate Acid Or Base.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. It can react with bases to. the standard answer is yes, because hx2sox4 h x 2 s o. Copper Ii Sulfate Acid Or Base.
From chemicaldb.netlify.app
Copper 2 sulfate with water heat Copper Ii Sulfate Acid Or Base the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). It can react with bases to. copper (ii) sulfate, also known. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Hydrated copper (II) sulphate crystals Stock Image C004/7690 Science Photo Library Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). It can react with bases to. . Copper Ii Sulfate Acid Or Base.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID9660645 Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. copper (ii) sulfate, also known as cupric sulfate,. Copper Ii Sulfate Acid Or Base.
From www.slideshare.net
Acids And Bases Copper Ii Sulfate Acid Or Base the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 − is a. It can react with bases to. copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Note, hydrogen can. Copper Ii Sulfate Acid Or Base.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used in medical and public Copper Ii Sulfate Acid Or Base in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). Note, hydrogen can not lose its only electron as then it. It can react with bases to. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. copper (ii) sulfate, also known as cupric. Copper Ii Sulfate Acid Or Base.
From www.youtube.com
Copper (II) Sulfate + Sodium Carbonate = (Double Displacement with Precipitate) YouTube Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. . Copper Ii Sulfate Acid Or Base.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate & copper (II) sulfate solution Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. Note, hydrogen can not lose its only electron as then it. if it is an acid, we base it's name on the ionic compound. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Anhydrous copper (II) sulphate Stock Image C014/6956 Science Photo Library Copper Ii Sulfate Acid Or Base to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Copper (II) sulphate forms Stock Image C004/7693 Science Photo Library Copper Ii Sulfate Acid Or Base the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. Note, hydrogen can not lose its only electron as then it. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. the standard answer is yes, because hx2sox4. Copper Ii Sulfate Acid Or Base.
From www.youtube.com
How to separate sand and copper(II) sulfate salt from its mixture Separation Methods YouTube Copper Ii Sulfate Acid Or Base Note, hydrogen can not lose its only electron as then it. in aqueous solutions, copper(ii) sulfate exhibits acidic properties due to the release of h+ ions from the sulfate ion (so₄²⁻). the standard answer is yes, because hx2sox4 h x 2 s o x 4 is a strong acid, hence sox4x2− s o x 4 x 2 −. Copper Ii Sulfate Acid Or Base.
From www.sciencephoto.com
Copper (II) Sulphate Stock Image C036/3376 Science Photo Library Copper Ii Sulfate Acid Or Base the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and bluestone. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. to tell if cuso4 (copper (ii) sulfate) forms an. Copper Ii Sulfate Acid Or Base.
From www.pw.live
Copper Sulfate Formula, Structure, Properties, Uses Copper Ii Sulfate Acid Or Base copper (ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. if it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. to tell if cuso4 (copper (ii) sulfate) forms an acidic, basic (alkaline), or. in. Copper Ii Sulfate Acid Or Base.