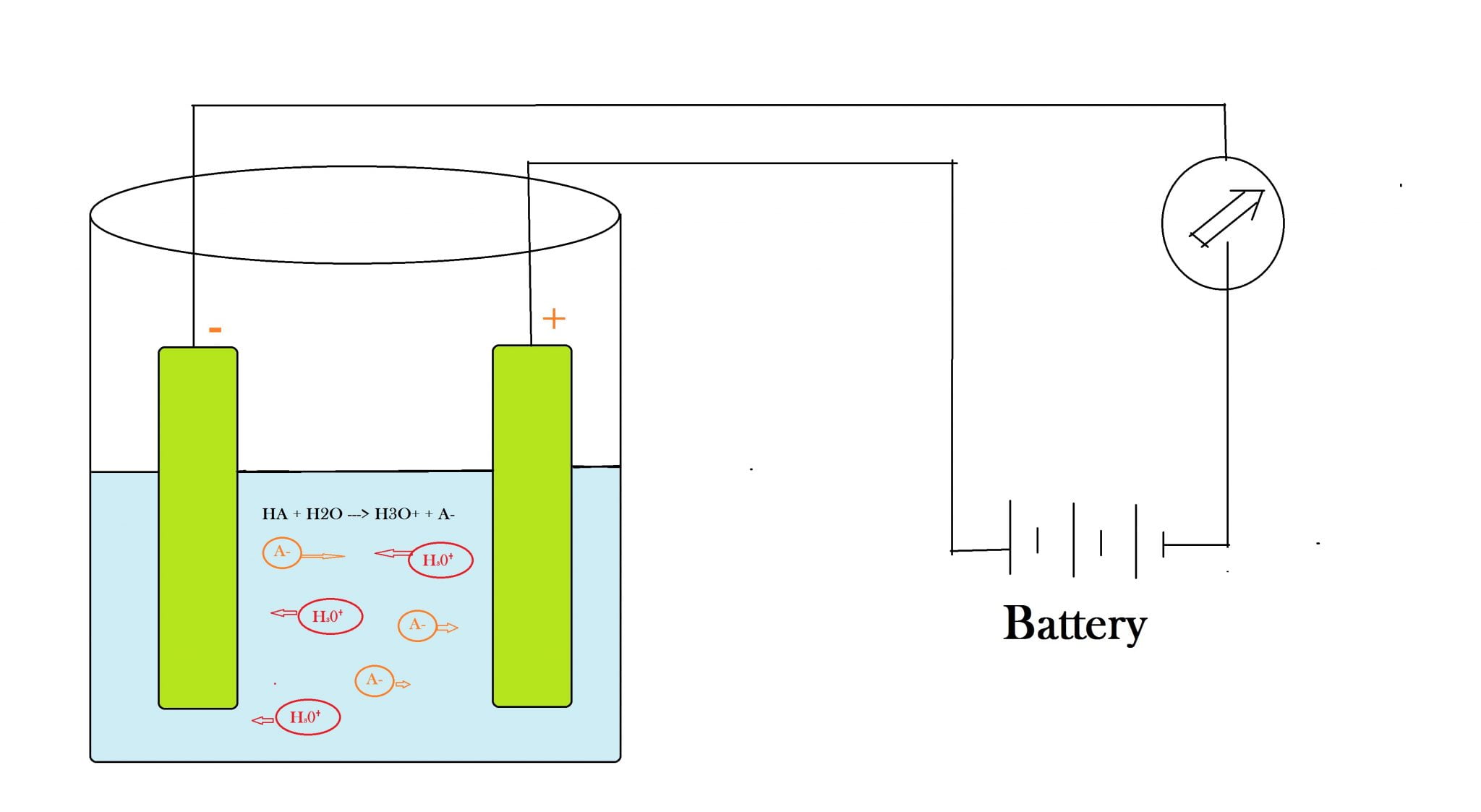
Electrical Conductivity Acids . electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester.
from studdy.in
the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid.
Why does an aqueous solution of acid conduct electricity? Studdy
Electrical Conductivity Acids strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related.
From www.thoughtco.com
Electrical Conductivity of Metals Electrical Conductivity Acids the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. in this experiment, students will. Electrical Conductivity Acids.
From www.researchgate.net
Electrical conductivity κ of phosphoric acid solutions vs. water... Download Scientific Diagram Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution.. Electrical Conductivity Acids.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions PowerPoint Presentation ID Electrical Conductivity Acids the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. . Electrical Conductivity Acids.
From slidetodoc.com
Strength of Acids and Bases Electrical Conductivity Acidic Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the conductivity of electrolytes (such as solutions of acids, alkalis and salts. Electrical Conductivity Acids.
From zoe-bogspotorr.blogspot.com
Electrical Conductivity of Acids and Bases Experiment Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. lewis acids and bases react. Electrical Conductivity Acids.
From www.slideserve.com
PPT Acids, Bases and Solutions Chapter 7 PowerPoint Presentation, free download ID3070308 Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. an acid or base which. Electrical Conductivity Acids.
From www.slideserve.com
PPT Substantially Conductive Polymers PowerPoint Presentation ID2067810 Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. an acid or base which. Electrical Conductivity Acids.
From mungfali.com
Electrical Conductivity Explained Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. in this experiment, students will determine the conductivity of different aqueous solutions. Electrical Conductivity Acids.
From imat.entermedschool.com
Higher electrical conductivity in acid and bases Practice Question Solving EnterMedSchool Electrical Conductivity Acids lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the conductivity of electrolytes (such as solutions of acids, alkalis. Electrical Conductivity Acids.
From slidetodoc.com
Strength of Acids and Bases Electrical Conductivity Acidic Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing. Electrical Conductivity Acids.
From www.youtube.com
Electrical conductivity of 100 acetic acid diluted Acetic acid solutions YouTube Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. lewis acids and bases react to create an adduct, a compound in which the acid and base. Electrical Conductivity Acids.
From www.youtube.com
Why aqueous Acids conduct electricity? Acid , Base and salts Animated lectureJatin academy Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the conductivity of electrolytes (such as solutions of acids,. Electrical Conductivity Acids.
From www.youtube.com
Electrical conductivity of Acids & Bases Random Topics Shorts 2 Shorta Sollanuna shorts Electrical Conductivity Acids lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. an acid or. Electrical Conductivity Acids.
From www.teachoo.com
[MCQ] In an attempt to demonstrate electrical conductivity through Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. lewis. Electrical Conductivity Acids.
From sciencenotes.org
What Is the Most Conductive Element? Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. strong acids and salts are strong electrolytes because they completely ionize (dissociate. Electrical Conductivity Acids.
From www.chegg.com
Solved Comment on the conductivity of the acids and bases Electrical Conductivity Acids lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of.. Electrical Conductivity Acids.
From byjus.com
the highest electrical conductivity from the following aqueous 1.0.1 M acetic acid 2.0.1M Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with. Electrical Conductivity Acids.
From chemistry.stackexchange.com
Why does the graph of the electrical conductivity of sulfuric acid/water solutions have this Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. an acid or base. Electrical Conductivity Acids.
From slideplayer.com
Strengths of Acids and Bases. Electrical Conductivity Acidic and basic solutions conduct Electrical Conductivity Acids the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related.. Electrical Conductivity Acids.
From www.chegg.com
2 Experiment 6 Electrical Conductivity Acids and Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function. Electrical Conductivity Acids.
From studylib.net
electrical conductivity of aqueous solutions Electrical Conductivity Acids electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the.. Electrical Conductivity Acids.
From www.researchgate.net
Dependence of the electrical conductivity of aqueous solutions of... Download Scientific Diagram Electrical Conductivity Acids electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. an. Electrical Conductivity Acids.
From www.slideserve.com
PPT The differences between strong and weak acids PowerPoint Presentation ID9722231 Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. an acid or base which strongly conducts electricity contains a large number of ions and is called. Electrical Conductivity Acids.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free download ID2139680 Electrical Conductivity Acids strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. an acid or. Electrical Conductivity Acids.
From studdy.in
Why does an aqueous solution of acid conduct electricity? Studdy Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. . Electrical Conductivity Acids.
From www.youtube.com
ACIDS Conduct Electricity! YouTube Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or. Electrical Conductivity Acids.
From www.teachoo.com
Why does aqueous solution of an acid conduct electricity? Class 10 Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. an acid or base. Electrical Conductivity Acids.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and NonElectrolytes Electrical Conductivity Acids lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong. Electrical Conductivity Acids.
From studylib.net
WS04 Electrical Conductivity Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. . Electrical Conductivity Acids.
From www.slideserve.com
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101 Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other dissolvents along with molten. an. Electrical Conductivity Acids.
From slideplayer.com
ACIDS AND BASES. ppt download Electrical Conductivity Acids the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. the conductivity of electrolytes (such as solutions of acids, alkalis and salts. Electrical Conductivity Acids.
From www.researchgate.net
Electrical conductivity of MNFG coated with different organic acids and... Download Scientific Electrical Conductivity Acids electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. lewis acids and bases react to create an adduct, a compound in which the acid and base have bonded by sharing the.. Electrical Conductivity Acids.
From www.teachoo.com
What do all Acids and Bases have in common? (with 5+ Points) Teachoo Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts. Electrical Conductivity Acids.
From www.researchgate.net
Results from measurements of electric conductivity and pH and... Download Scientific Diagram Electrical Conductivity Acids an acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid. strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the conductivity of electrolytes (such as solutions of acids, alkalis and salts in water and other. Electrical Conductivity Acids.
From zoe-bogspotorr.blogspot.com
Electrical Conductivity of Acids and Bases Experiment Electrical Conductivity Acids in this experiment, students will determine the conductivity of different aqueous solutions using a conductivity tester. electrical conductivity (ec) and ph are two fundamental properties of solutions that are closely related. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. lewis acids and bases react. Electrical Conductivity Acids.