Point Equivalent Definition . The term end point is where the indicator changes. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. When solutions of some polyprotic acids are. In other words, while titrating, it. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation.
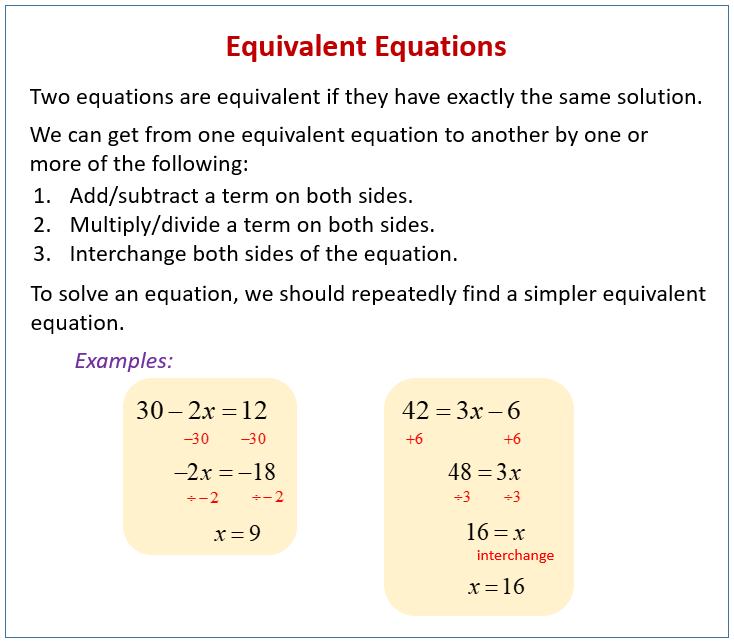
from www.onlinemathlearning.com
The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term end point is where the indicator changes. When solutions of some polyprotic acids are. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In other words, while titrating, it. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated.
Equivalent Equations (solutions, examples, worksheets, videos, games
Point Equivalent Definition The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term end point is where the indicator changes. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. When solutions of some polyprotic acids are. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation.
From www.sciencemotive.com
Differentiate Between Endpoint and Equivalence Point ScienceMotive Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term end point is where the indicator changes. The term equivalence point means. Point Equivalent Definition.
From gamesmartz.com
Equivalence Point Definition & Image GameSmartz Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When solutions of some polyprotic acids are. The equivalence point is the point at which titrant has been added in exactly. Point Equivalent Definition.
From www.numerade.com
SOLVED Define two points (x1, y1) and (x2, y2) in R2 to be equivalent Point Equivalent Definition The term end point is where the indicator changes. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In other words, while titrating, it. When solutions of some polyprotic acids are. The equivalence point of a chemical reaction is the point at which equal quantities of. Point Equivalent Definition.
From www.slideshare.net
Vocab maths 8_ratios_and_rates Point Equivalent Definition The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term equivalence point means that the solutions have been. Point Equivalent Definition.
From slideplayer.com
Equivalence Point. ppt download Point Equivalent Definition When solutions of some polyprotic acids are. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point is the point at which titrant has been added in. Point Equivalent Definition.
From slideplayer.com
1 Copyright, 1996 © Dale Carnegie & Associates, Inc. Chapter 2 Logic Point Equivalent Definition When solutions of some polyprotic acids are. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the. Point Equivalent Definition.
From www.youtube.com
Finding equivalence point for Monoprotic acid YouTube Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term end point is where the indicator changes. When solutions of some polyprotic acids are. In other words, while titrating, it. The. Point Equivalent Definition.
From thecontentauthority.com
Equivalence Point vs Endpoint Meaning And Differences Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. When solutions of some polyprotic acids are. In other words, while titrating, it. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant.. Point Equivalent Definition.
From www.vrogue.co
Understanding Equivalent Equations In Algebra vrogue.co Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The main difference between equivalence and endpoint is that the equivalence point is a point. Point Equivalent Definition.
From www.pdfprof.com
point d'équivalence définition Point Equivalent Definition The term end point is where the indicator changes. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The main difference between equivalence and endpoint is that. Point Equivalent Definition.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Point Equivalent Definition The term end point is where the indicator changes. When solutions of some polyprotic acids are. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the. Point Equivalent Definition.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Point Equivalent Definition The term end point is where the indicator changes. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In other words, while titrating, it. The. Point Equivalent Definition.
From www.youtube.com
How to Calculate the Volume of Titrant Needed to Reach Equivalence Point Equivalent Definition The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In other words, while titrating, it. When solutions of some polyprotic acids are. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term end. Point Equivalent Definition.
From www.slideserve.com
PPT Order Structure, Correspondence, and Shape Based Categories Point Equivalent Definition In other words, while titrating, it. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. When solutions of some polyprotic acids are. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The main difference between equivalence and endpoint is that the. Point Equivalent Definition.
From askfilo.com
Find the equivalent force and equivalent moment at a point ' O '. The rad.. Point Equivalent Definition In other words, while titrating, it. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. When solutions of some polyprotic acids are. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the point at. Point Equivalent Definition.
From www.researchgate.net
The definition of the equivalent point. Download Scientific Diagram Point Equivalent Definition The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. When solutions of some polyprotic acids are. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term equivalence point means that the solutions have. Point Equivalent Definition.
From www.onlinemathlearning.com
Equivalent Equations (solutions, examples, worksheets, videos, games Point Equivalent Definition In other words, while titrating, it. When solutions of some polyprotic acids are. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The. Point Equivalent Definition.
From sciencenotes.org
Equivalent Equations in Algebra Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent. Point Equivalent Definition.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Point Equivalent Definition When solutions of some polyprotic acids are. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term end point is where the indicator changes.. Point Equivalent Definition.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When solutions of some polyprotic acids are. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term equivalence point means that the solutions have been mixed in exactly the right proportions according. Point Equivalent Definition.
From www.slideserve.com
PPT CHAPTER 6 TITRIMETRIC METHODS OF ANALYSIS PowerPoint Presentation Point Equivalent Definition In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In other words, while titrating, it. The main difference between equivalence and endpoint is that the. Point Equivalent Definition.
From www.slideserve.com
PPT Order Structure, Correspondence, and Shape Based Categories Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at. Point Equivalent Definition.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download Point Equivalent Definition In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term equivalence point means that the solutions have been mixed in exactly the right proportions. Point Equivalent Definition.
From www.splashlearn.com
What is a Point in Math? Definition, Properties, Uses, Examples Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. When solutions of some polyprotic acids are. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants. Point Equivalent Definition.
From www.numerade.com
16. The four motion diagrams below show an initial point (0) and final Point Equivalent Definition In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. In other words, while titrating, it. The main difference between equivalence and endpoint is that the equivalence point is a point where the. Point Equivalent Definition.
From definitionghw.blogspot.com
Definition Of Equivalence Point Chemistry DEFINITION GHW Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the point at which titrant has been added in exactly the right quantity to. Point Equivalent Definition.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. In other words, while. Point Equivalent Definition.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The term end point is where the indicator changes. In other words, while titrating, it. The equivalence. Point Equivalent Definition.
From www.pw.live
Difference Between Endpoint And Equivalence Point Point Equivalent Definition The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. When solutions of some polyprotic acids are. In summary, the equivalence point is. Point Equivalent Definition.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. When solutions of some. Point Equivalent Definition.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point Point Equivalent Definition The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. The term end point is where the indicator changes. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of. Point Equivalent Definition.
From www.youtube.com
Position Vector of a Point Definition, Example, Class 11 YouTube Point Equivalent Definition The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The main difference between equivalence and endpoint is that the equivalence point is. Point Equivalent Definition.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Point Equivalent Definition The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. In summary, the equivalence point is a theoretical concept representing. Point Equivalent Definition.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Point Equivalent Definition The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The term end point is where the indicator changes. When solutions of some polyprotic acids are. In other words, while titrating, it. The main difference between equivalence and endpoint is that the equivalence. Point Equivalent Definition.
From study.com
Finding the Equivalence Point Titration & Examples Lesson Point Equivalent Definition In summary, the equivalence point is a theoretical concept representing the stoichiometrically equivalent amounts of reactants, calculated. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant. The equivalence point of a chemical reaction is the point at which equal quantities of reactants. Point Equivalent Definition.