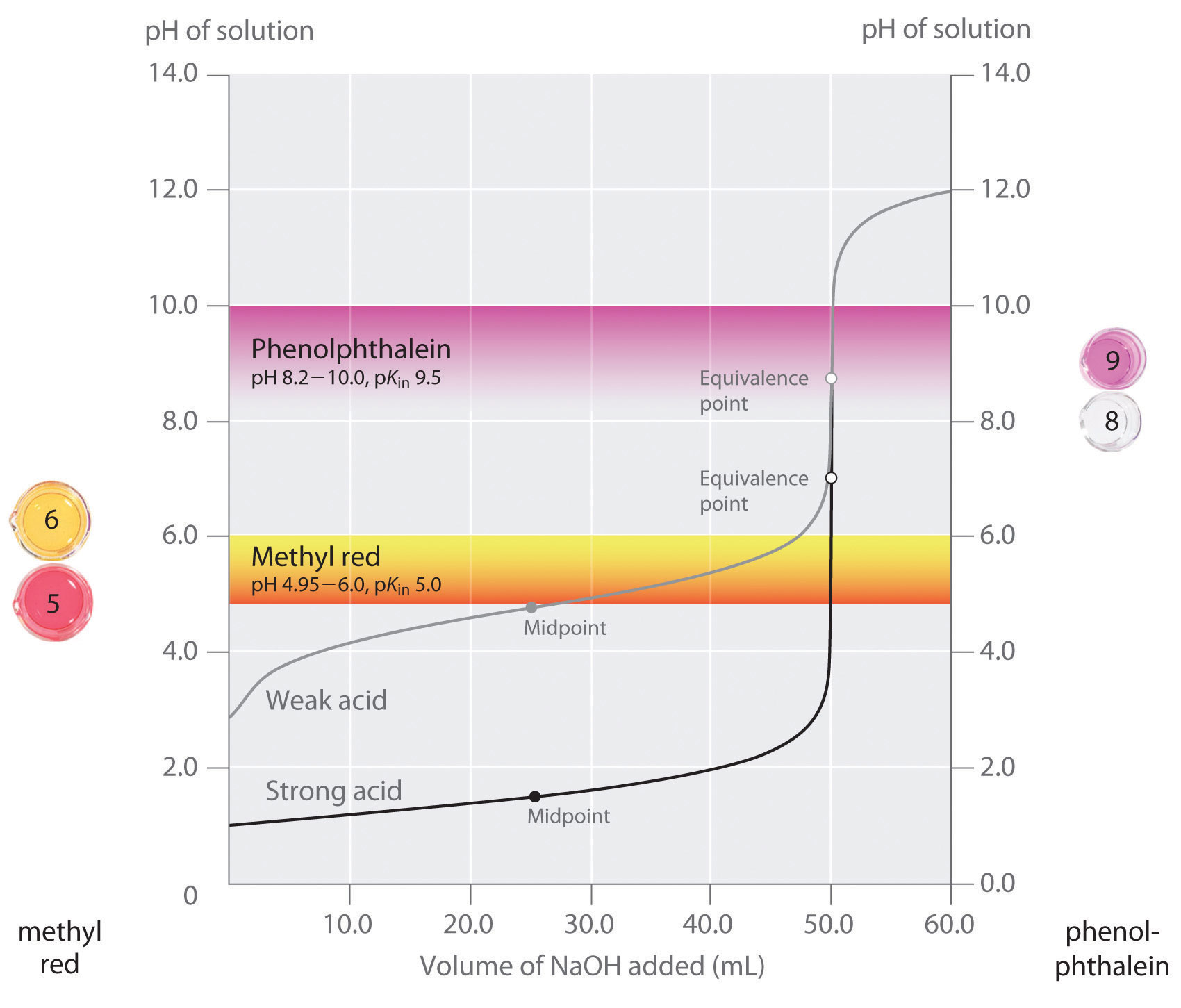
Indicator For Strong Acid And Base . strong acid vs. Any of the three indicators will. when titrating a strong acid or base, the endpoint should occur at a ph of 7. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. titrate a weak acid using an indicator that changes under slightly alkaline conditions. This page assumes that you. The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak base using an indicator that changes color at a slightly. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely.
from chem.libretexts.org
The next diagram shows the ph curve for adding a strong acid to a strong base. Any of the three indicators will. Titrate a weak base using an indicator that changes color at a slightly. strong acid vs. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. This page assumes that you. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline conditions. when titrating a strong acid or base, the endpoint should occur at a ph of 7.
17.3 AcidBase Indicators Chemistry LibreTexts
Indicator For Strong Acid And Base Any of the three indicators will. when titrating a strong acid or base, the endpoint should occur at a ph of 7. titrate a weak acid using an indicator that changes under slightly alkaline conditions. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak base using an indicator that changes color at a slightly. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. This page assumes that you. strong acid vs. Any of the three indicators will.
From www.slideserve.com
PPT More Acid/Base Equilibria !!! 17.1 17.3 PowerPoint Presentation Indicator For Strong Acid And Base This page assumes that you. when titrating a strong acid or base, the endpoint should occur at a ph of 7. strong acid vs. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. Any of the three. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation, free download Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. The next diagram shows the ph curve for adding a strong acid to a strong base. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. titration of. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Indicator For Strong Acid And Base This page assumes that you. Any of the three indicators will. strong acid vs. Titrate a weak base using an indicator that changes color at a slightly. The next diagram shows the ph curve for adding a strong acid to a strong base. in general, for titrations of strong acids with strong bases (and vice versa), any indicator. Indicator For Strong Acid And Base.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Indicator For Strong Acid And Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the ph curve for adding a strong acid to a strong base.. Indicator For Strong Acid And Base.
From slideplayer.com
Properties of Acids and Bases ppt download Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. This page assumes that you. titrate a weak acid using an indicator that changes under slightly alkaline conditions.. Indicator For Strong Acid And Base.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. when titrating a strong acid or base, the endpoint should occur at a ph of 7. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. Titrate a weak. Indicator For Strong Acid And Base.
From study.com
AcidBase Indicator Definition, Concept & Examples Lesson Indicator For Strong Acid And Base strong acid vs. titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that changes color at a slightly. This page assumes that you. Any of the three indicators will. titration of a strong acid with a strong base is the simplest of the four types of. Indicator For Strong Acid And Base.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry Indicator For Strong Acid And Base in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak base using an. Indicator For Strong Acid And Base.
From www.dreamstime.com
Ph Scale. Infographic Acidbase Balance. Scale for Chemical Analysis Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline conditions.. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT ACIDBASE INDICATORS PowerPoint Presentation, free download ID Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. This page assumes that you. The next diagram shows the ph curve for adding a strong acid to a strong base. titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6973259 Indicator For Strong Acid And Base This page assumes that you. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. The next diagram shows the ph curve for adding a strong acid to a strong base. strong acid vs. titrate a weak acid using an indicator that changes under. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Indicator For Strong Acid And Base This page assumes that you. strong acid vs. titrate a weak acid using an indicator that changes under slightly alkaline conditions. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. in general, for titrations of strong. Indicator For Strong Acid And Base.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. This page assumes that you. strong acid vs. Titrate a weak base using an indicator that changes color at. Indicator For Strong Acid And Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Strong Acid And Base in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. when titrating a strong acid or base, the endpoint should occur at a ph of 7. Any of the three indicators will. Titrate a weak base using an indicator that changes color at a slightly.. Indicator For Strong Acid And Base.
From cehfduiq.blob.core.windows.net
Indicators Are Acids And Bases at Wilfred Rollins blog Indicator For Strong Acid And Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. The next diagram shows the ph curve for adding a strong acid to a strong base. strong acid vs. Any of the three indicators will. Titrate a weak base. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Acids & Alkalis (Bases) PowerPoint Presentation, free download Indicator For Strong Acid And Base strong acid vs. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. Any of. Indicator For Strong Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator For Strong Acid And Base Titrate a weak base using an indicator that changes color at a slightly. This page assumes that you. The next diagram shows the ph curve for adding a strong acid to a strong base. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. titrate. Indicator For Strong Acid And Base.
From davidswart.wordpress.com
Acid and Base Lab with Report The Art of Science Indicator For Strong Acid And Base titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that changes color at a slightly. The next diagram shows the ph curve for adding a strong acid to a strong base. strong acid vs. when titrating a strong acid or base, the endpoint should occur at. Indicator For Strong Acid And Base.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator For Strong Acid And Base Any of the three indicators will. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. strong acid vs. Titrate a weak base using an indicator that changes color at a slightly. when titrating a strong acid or. Indicator For Strong Acid And Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak base using an indicator that changes color at a slightly. This page assumes that you. titrate a weak acid using an indicator that changes under slightly alkaline conditions. in general, for titrations of strong acids with strong bases (and. Indicator For Strong Acid And Base.
From www.slideshare.net
Acid base titration Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. Any of the three indicators will. strong acid vs. titrate a weak acid using an indicator that. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. This page assumes that you. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. Titrate a weak base using an indicator that changes color at a slightly. when. Indicator For Strong Acid And Base.
From dxoqotyly.blob.core.windows.net
Acid Base Titration Indicator Example at Amanda Byrne blog Indicator For Strong Acid And Base Any of the three indicators will. This page assumes that you. strong acid vs. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline conditions. . Indicator For Strong Acid And Base.
From www.youtube.com
Acid Base Indicators Introduction Acids and Bases Chemistry Practice Indicator For Strong Acid And Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that changes color at a slightly. This page assumes. Indicator For Strong Acid And Base.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Indicator For Strong Acid And Base Titrate a weak base using an indicator that changes color at a slightly. This page assumes that you. The next diagram shows the ph curve for adding a strong acid to a strong base. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. when. Indicator For Strong Acid And Base.
From www.youtube.com
acids,bases and salts indicators class10 by poonam malhotra YouTube Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. titrate a weak acid using an indicator that changes under slightly alkaline. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Indicator For Strong Acid And Base Titrate a weak base using an indicator that changes color at a slightly. titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the ph curve for adding a strong acid to a strong base. strong acid vs. Any of the three indicators will. This page assumes that you. when. Indicator For Strong Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator For Strong Acid And Base Titrate a weak base using an indicator that changes color at a slightly. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. titrate a weak acid using an indicator that changes under slightly alkaline conditions. strong acid vs. titration of a strong. Indicator For Strong Acid And Base.
From cerjqebi.blob.core.windows.net
Indicators For Acid Or Base at Lily Holtz blog Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. Titrate a weak base using an indicator that changes color at a slightly.. Indicator For Strong Acid And Base.
From www.sigmaaldrich.com
Acid and Base Chart — Table of Acids & Bases Indicator For Strong Acid And Base strong acid vs. when titrating a strong acid or base, the endpoint should occur at a ph of 7. Any of the three indicators will. This page assumes that you. The next diagram shows the ph curve for adding a strong acid to a strong base. titrate a weak acid using an indicator that changes under slightly. Indicator For Strong Acid And Base.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Strong Acid And Base The next diagram shows the ph curve for adding a strong acid to a strong base. titrate a weak acid using an indicator that changes under slightly alkaline conditions. This page assumes that you. strong acid vs. Titrate a weak base using an indicator that changes color at a slightly. Any of the three indicators will. titration. Indicator For Strong Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. Titrate a weak base using an indicator that changes color at a slightly. strong acid vs. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and. Indicator For Strong Acid And Base.
From slideplayer.com
Properties of Acids and Bases ppt download Indicator For Strong Acid And Base Titrate a weak base using an indicator that changes color at a slightly. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and. strong acid vs. titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the. Indicator For Strong Acid And Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 Indicator For Strong Acid And Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. Any of the three indicators will. in general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and.. Indicator For Strong Acid And Base.
From mavink.com
Ph Scale Weak And Strong Indicator For Strong Acid And Base when titrating a strong acid or base, the endpoint should occur at a ph of 7. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely. strong acid vs. Any of the three indicators will. titrate a. Indicator For Strong Acid And Base.