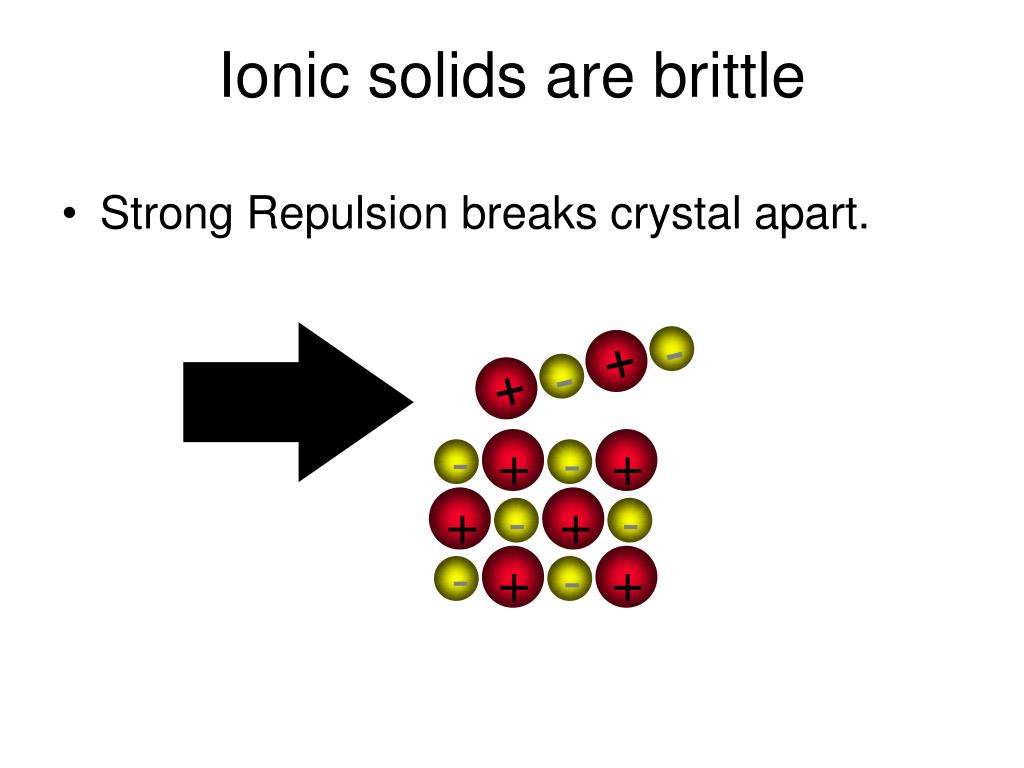
Why Do Ionic Compounds Be Brittle . However, when that happens, it brings ions of the same charge next to one another (see below). When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are strong and hard. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard, but brittle.
from www.slideserve.com
In summary, brittleness is not a property uniquely associated with ionic compounds. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are strong and hard. Ionic compounds are brittle because they have a crystal lattice structure. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. When an external force is applied, it can shift the layers of ions, causing.
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint
Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. In summary, brittleness is not a property uniquely associated with ionic compounds. However, when that happens, it brings ions of the same charge next to one another (see below). It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are strong and hard. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle.
From slideplayer.com
Ionic and Metallic Bonds ppt download Why Do Ionic Compounds Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are strong and hard. Explain why most ionic compounds are strong and hard, yet brittle. When an external force is applied, it can. Why Do Ionic Compounds Be Brittle.
From www.youtube.com
Why ionic solids are brittle? YouTube Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions. Why Do Ionic Compounds Be Brittle.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Why Do Ionic Compounds Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are brittle because they have a crystal lattice structure. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions. Why Do Ionic Compounds Be Brittle.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Do Ionic Compounds Be Brittle In summary, brittleness is not a property uniquely associated with ionic compounds. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Why Do Ionic Compounds Be Brittle.
From slidereverse.blogspot.com
How Do Ions Form Ionic Bonds Why Do Ionic Compounds Be Brittle Ionic compounds are brittle because they have a crystal lattice structure. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. However, when that happens, it brings ions of the same charge next to one. Why Do Ionic Compounds Be Brittle.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Why Do Ionic Compounds Be Brittle Ionic compounds are strong and hard. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer,. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Do Ionic Compounds Be Brittle Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. When an external force is applied, it can shift the layers of ions,. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID5979988 Why Do Ionic Compounds Be Brittle Ionic compounds are strong and hard. Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers of ions, causing. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are brittle due to the strong bond between the positive and. Why Do Ionic Compounds Be Brittle.
From www.doubtnut.com
Explain why ionic compounds are hard and brittle? Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. In summary, brittleness is not a property uniquely associated with ionic compounds. When an external force is applied, it can shift the layers. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bonds and Compounds ppt download Why Do Ionic Compounds Be Brittle Ionic compounds are brittle because they have a crystal lattice structure. In summary, brittleness is not a property uniquely associated with ionic compounds. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds are strong and hard. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because. Why Do Ionic Compounds Be Brittle.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Why Do Ionic Compounds Be Brittle Ionic compounds are strong and hard. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are. Why Do Ionic Compounds Be Brittle.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Why Do Ionic Compounds Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. However, when that happens, it brings ions of the same charge next to one another (see below). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely.. Why Do Ionic Compounds Be Brittle.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers of ions, causing. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. Explain why most ionic compounds are strong and hard,. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Why Do Ionic Compounds Be Brittle In summary, brittleness is not a property uniquely associated with ionic compounds. However, when that happens, it brings ions of the same charge next to one another (see below). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. It takes a large amount of mechanical force, such as striking. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint Why Do Ionic Compounds Be Brittle However, when that happens, it brings ions of the same charge next to one another (see below). When an external force is applied, it can shift the layers of ions, causing. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic and Covalent Bonds ppt download Why Do Ionic Compounds Be Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Explain why most ionic compounds are strong and hard, yet brittle. It. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bonding/Naming. ppt download Why Do Ionic Compounds Be Brittle Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bonds. ppt download Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. Explain why most ionic compounds are strong and hard, yet brittle. However, when that happens, it brings ions of the same charge next to one another (see below). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move. Why Do Ionic Compounds Be Brittle.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are brittle due to the strong bond between the positive and negative. Why Do Ionic Compounds Be Brittle.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Why Do Ionic Compounds Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. When an external force is applied, it can shift. Why Do Ionic Compounds Be Brittle.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. When an external force is applied, it can shift the layers of ions, causing. However, when that happens, it brings ions of the same charge next to one another (see below). Once dissolved or melted, ionic compounds are excellent conductors of electricity. Why Do Ionic Compounds Be Brittle.
From slidetodoc.com
Lesson 3 Properties of Ionic Compounds Learning Objectives Why Do Ionic Compounds Be Brittle However, when that happens, it brings ions of the same charge next to one another (see below). It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Why Do Ionic Compounds Be Brittle In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds are. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Properties of Ionic Bonds PowerPoint Presentation, free download Why Do Ionic Compounds Be Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds are brittle because they have a crystal lattice structure. In summary, brittleness is not a property uniquely associated with ionic compounds.. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic and Covalent Compounds ppt download Why Do Ionic Compounds Be Brittle Explain why most ionic compounds are strong and hard, yet brittle. When an external force is applied, it can shift the layers of ions, causing. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are strong and hard. Ionic. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bonding. ppt download Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are strong and hard. Ionic compounds are generally hard, but brittle. Once dissolved. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Why Do Ionic Compounds Be Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are strong and hard. Ionic compounds are generally hard, but brittle. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions. Why Do Ionic Compounds Be Brittle.
From www.youtube.com
Why are ionic compounds hard and brittle? YouTube Why Do Ionic Compounds Be Brittle Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. When an external force is applied, it can shift the layers of ions, causing. However, when that happens, it brings ions of the same charge next to one another (see below). It takes a large amount of mechanical force, such. Why Do Ionic Compounds Be Brittle.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard, but brittle. However, when that happens, it brings ions of the same charge. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Why Do Ionic Compounds Be Brittle Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard. In summary, brittleness is not a property uniquely associated with ionic compounds. However, when that happens, it brings ions of the same charge next to one another. Why Do Ionic Compounds Be Brittle.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are strong and hard. Ionic compounds are brittle because they. Why Do Ionic Compounds Be Brittle.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. Ionic compounds are generally hard, but brittle. In summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. However, when that happens,. Why Do Ionic Compounds Be Brittle.
From www.youtube.com
GCSE Chemistry 19 Why do Ionic Compounds have High Melting Points Why Do Ionic Compounds Be Brittle Ionic compounds are generally hard, but brittle. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are strong and hard. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. However, when that happens, it brings ions of the. Why Do Ionic Compounds Be Brittle.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Why Do Ionic Compounds Be Brittle When an external force is applied, it can shift the layers of ions, causing. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are strong and hard. However, when that happens, it brings ions of the same charge next to one another (see below). It takes a large amount of mechanical force, such as striking a. Why Do Ionic Compounds Be Brittle.