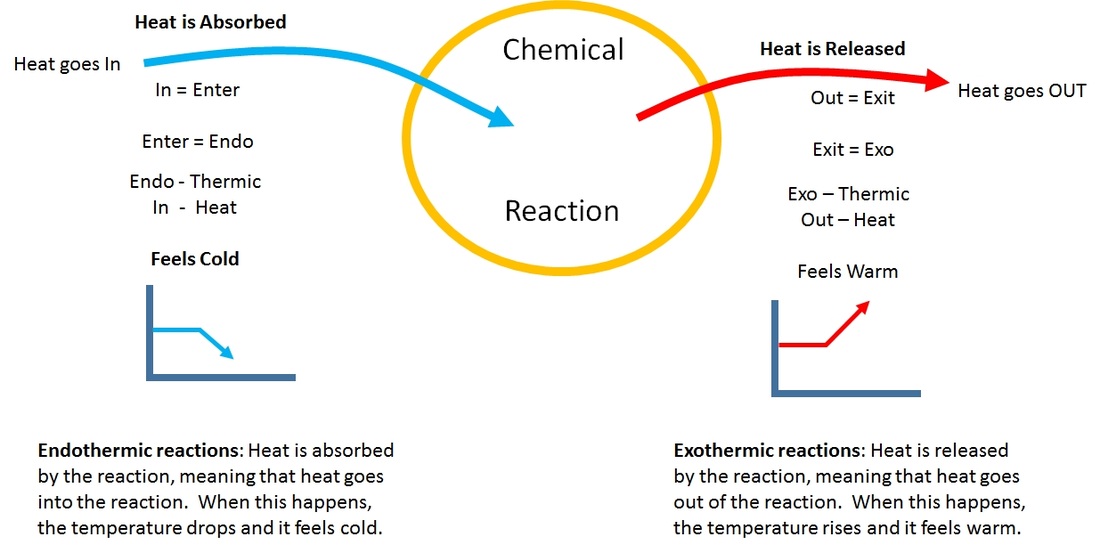
Endothermic Reaction And Solution . chemical reactions that release energy are called exothermic. You can find citric acid and baking soda at the grocery. what is an endothermic reaction? In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic reactions, more energy is. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Baking soda (sodium bicarbonate) water. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.
from vhmsscience.weebly.com
in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In exothermic reactions, more energy is released when the bonds are formed in the products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. You can find citric acid and baking soda at the grocery. what is an endothermic reaction? Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. In endothermic reactions, more energy is. chemical reactions that release energy are called exothermic. chemical reactions that absorb (or use) energy overall are called endothermic. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE
Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. chemical reactions that absorb (or use) energy overall are called endothermic. You can find citric acid and baking soda at the grocery. In endothermic reactions, more energy is. Baking soda (sodium bicarbonate) water. chemical reactions that release energy are called exothermic. what is an endothermic reaction? In exothermic reactions, more energy is released when the bonds are formed in the products. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a.
From learnphysics-dhruv.blogspot.com
Physics Learn Change in Chemical reactions Exothermic OR Endothermic GSEB Science 8 to 12 Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic reactions, more energy is. These reactions lower the. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Endothermic Reaction And Solution what is an endothermic reaction? In endothermic reactions, more energy is. You can find citric acid and baking soda at the grocery. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In exothermic reactions, more energy is released when the bonds are formed in the products. Baking soda (sodium bicarbonate) water. chemical reactions. Endothermic Reaction And Solution.
From materialmediabrindle.z21.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Endothermic Reaction And Solution describe three ways to speed up a reaction. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. You can find citric acid and baking soda at the grocery. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Differentiate between. Endothermic Reaction And Solution.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. what is an endothermic reaction? Baking soda (sodium bicarbonate) water. In endothermic reactions, more energy is. chemical reactions that release energy are called exothermic. You can. Endothermic Reaction And Solution.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Illustration Adobe Stock Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. what is an endothermic reaction? Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. describe three ways to speed up a reaction. You can find citric acid and baking. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Endothermic Reaction And Solution describe three ways to speed up a reaction. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that absorb (or use) energy overall are called endothermic. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and. Endothermic Reaction And Solution.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes Endothermic Reaction And Solution what is an endothermic reaction? These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Baking soda (sodium bicarbonate) water. chemical reactions that release energy are called exothermic. In endothermic reactions, more energy is. chemical reactions that absorb (or use) energy overall are called endothermic. in endothermic and exothermic reactions, energy can. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Endothermic Reaction And Solution describe three ways to speed up a reaction. chemical reactions that release energy are called exothermic. what is an endothermic reaction? chemical reactions that absorb (or use) energy overall are called endothermic. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Endothermic reactions are chemical reactions in which the reactants absorb. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION 5 1 exothermic and endothermic reactions Studypool Endothermic Reaction And Solution Baking soda (sodium bicarbonate) water. describe three ways to speed up a reaction. In endothermic reactions, more energy is. chemical reactions that release energy are called exothermic. chemical reactions that absorb (or use) energy overall are called endothermic. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. You. Endothermic Reaction And Solution.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Reaction And Solution chemical reactions that release energy are called exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. describe three ways to speed up a reaction. chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic reactions, more energy is. Endothermic reactions are chemical. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION 36 exothermic and endothermic reactions pptx Studypool Endothermic Reaction And Solution In exothermic reactions, more energy is released when the bonds are formed in the products. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect. Endothermic Reaction And Solution.
From treatybottle13.pythonanywhere.com
Fantastic 10 Examples Of Endothermic Reactions With Equations Edexcel Igcse Further Pure Endothermic Reaction And Solution describe three ways to speed up a reaction. chemical reactions that absorb (or use) energy overall are called endothermic. chemical reactions that release energy are called exothermic. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on. Endothermic Reaction And Solution.
From www.youtube.com
31 Endothermic and Exothermic Processes Solutions YouTube Endothermic Reaction And Solution In endothermic reactions, more energy is. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. chemical reactions that absorb (or use) energy overall are called endothermic. what is an endothermic reaction? chemical reactions that release energy are called exothermic. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions. Endothermic Reaction And Solution.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Endothermic Reaction And Solution In exothermic reactions, more energy is released when the bonds are formed in the products. what is an endothermic reaction? You can find citric acid and baking soda at the grocery. Baking soda (sodium bicarbonate) water. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Endothermic Reaction And Solution chemical reactions that absorb (or use) energy overall are called endothermic. chemical reactions that release energy are called exothermic. what is an endothermic reaction? describe three ways to speed up a reaction. Baking soda (sodium bicarbonate) water. In exothermic reactions, more energy is released when the bonds are formed in the products. These reactions lower the. Endothermic Reaction And Solution.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Chemistry Endothermic Reaction And Solution what is an endothermic reaction? in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Baking soda. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. chemical reactions that release energy are called exothermic. You can find citric acid and baking soda at the grocery. These reactions. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reaction Studypool Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. describe three ways to speed up a reaction. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Endothermic and Exothermic Reactions Studypool Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. describe three ways to speed up a reaction. You can find citric acid and baking soda at the grocery. Baking soda (sodium bicarbonate) water. chemical reactions. Endothermic Reaction And Solution.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. what is an endothermic reaction? In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that absorb (or use) energy overall are called endothermic. chemical reactions that release energy are. Endothermic Reaction And Solution.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction And Solution Baking soda (sodium bicarbonate) water. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that absorb (or use) energy. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Endothermic Reaction And Solution Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. what is an endothermic reaction? In endothermic reactions, more energy is. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on. Endothermic Reaction And Solution.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. chemical reactions that absorb (or use) energy overall are called endothermic. In exothermic. Endothermic Reaction And Solution.
From nathalianewsdeleon.blogspot.com
Explain the Difference Between Exothermic and Endothermic Reactions Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. what is an endothermic reaction? You can find citric acid and baking soda at the grocery. chemical reactions that release energy are called exothermic. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Chemistry exothermic endothermic reactions Studypool Endothermic Reaction And Solution chemical reactions that absorb (or use) energy overall are called endothermic. You can find citric acid and baking soda at the grocery. In exothermic reactions, more energy is released when the bonds are formed in the products. what is an endothermic reaction? Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each. Endothermic Reaction And Solution.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Equations Part 2 FlexiPrep Endothermic Reaction And Solution In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Baking soda (sodium bicarbonate) water. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction And Solution.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction And Solution what is an endothermic reaction? In exothermic reactions, more energy is released when the bonds are formed in the products. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. chemical reactions that release energy are called exothermic. In endothermic reactions, more energy is. Endothermic reactions are chemical. Endothermic Reaction And Solution.
From www.studypool.com
SOLUTION Exothermic and Endothermic Reactions GCSE Chemistry (5070) Studypool Endothermic Reaction And Solution In endothermic reactions, more energy is. what is an endothermic reaction? chemical reactions that release energy are called exothermic. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In exothermic reactions, more energy is released. Endothermic Reaction And Solution.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction And Solution in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic reactions, more energy is. chemical reactions that absorb (or use) energy overall are called endothermic. In exothermic reactions, more. Endothermic Reaction And Solution.
From www.doubtnut.com
An endothermic reaction is represented by the graph Endothermic Reaction And Solution You can find citric acid and baking soda at the grocery. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. what is an endothermic reaction? In endothermic reactions, more energy is. chemical reactions that absorb (or use) energy overall are called endothermic. chemical reactions that release. Endothermic Reaction And Solution.
From exolcytin.blob.core.windows.net
Endothermic Solution Process at Wilfred Hill blog Endothermic Reaction And Solution what is an endothermic reaction? In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that absorb (or use) energy overall are called endothermic. You can find citric acid and baking soda at the grocery. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each. Endothermic Reaction And Solution.
From chart-studio.plotly.com
Endothermic and Exothermic Reactions Graph scatter chart made by Jacey_m plotly Endothermic Reaction And Solution In endothermic reactions, more energy is. You can find citric acid and baking soda at the grocery. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. chemical reactions that absorb (or use) energy overall are called. Endothermic Reaction And Solution.
From studylibraryimburse.z22.web.core.windows.net
Endothermic Vs Exothermic Examples Chemistry Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. chemical reactions that release energy are called. Endothermic Reaction And Solution.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Solution describe three ways to speed up a reaction. what is an endothermic reaction? Baking soda (sodium bicarbonate) water. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. chemical reactions that absorb (or use) energy overall are called endothermic. You can find citric acid and baking soda at the. Endothermic Reaction And Solution.
From cexqwbtk.blob.core.windows.net
Endothermic Reaction Synthesis at John Dixon blog Endothermic Reaction And Solution Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. In endothermic reactions, more energy is. Baking soda (sodium bicarbonate) water. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the. Endothermic Reaction And Solution.