Indicator Reagent Definition . Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. They are usually weak acids or bases, but their. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to.
from mobbycorp.com
We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. Indicators are substances whose solutions change color due to changes in ph. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its.
pH & Reagent Indicators Mobby Business Corporation
Indicator Reagent Definition They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. They are usually weak acids or bases, but their. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its.
From www.pinterest.com
Colourful Chemistry Chemistry of UNIVERSAL INDICATOR Chemistry education, Chemistry lessons Indicator Reagent Definition As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. An indicator is a weak acid that ionizes within a known ph range, usually. Indicator Reagent Definition.
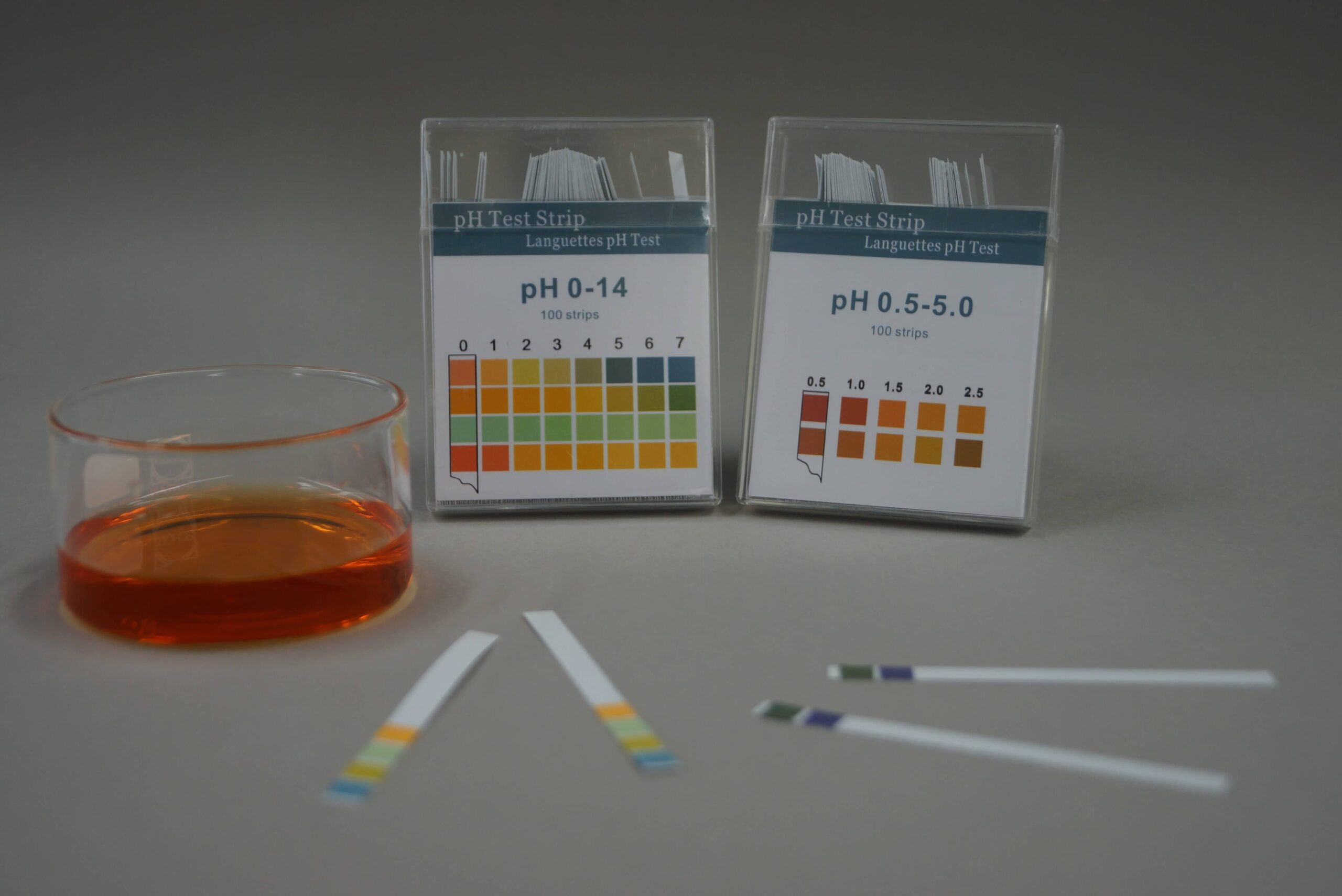
From fyowmwwcv.blob.core.windows.net
Indicator In Chemical Equation at Jeanne Cooney blog Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. Indicators are substances whose solutions change color due to changes in ph. As nouns the difference between reagent and indicator. Indicator Reagent Definition.
From www.youtube.com
Indicators Definition, Types, Examples and Facts 10th Class Chemistry Chapter Acids Bases and Indicator Reagent Definition Indicators are substances whose solutions change color due to changes in ph. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. A reagent which is termed as titrant or titrator is prepared on the. Indicator Reagent Definition.
From www.qprgllc.com
Chemical Indicators What to Use, When to Use Them, and What’s the Difference between Them All Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a. Indicator Reagent Definition.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. Indicators are substances whose solutions change color due to changes in ph. We can represent the protonated form of. Indicator Reagent Definition.
From www.storyboardthat.com
Indicators of a Chemical Reaction Storyboard by oliversmith Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence. Indicator Reagent Definition.
From sciencenotes.org
What Is a Reagent? Definition and Examples Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. Chemical indicator, any substance that gives a visible sign, usually by a color change, of. Indicator Reagent Definition.
From mobbycorp.com
pH & Reagent Indicators Mobby Business Corporation Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical. Indicator Reagent Definition.
From www.foxteach.co.uk
CHEMISTRY Indicator Reagent Definition As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard. Indicator Reagent Definition.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Indicator Reagent Definition We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. As nouns the difference between. Indicator Reagent Definition.
From www.youtube.com
Indicators Chemical Reaction YouTube Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually. Indicator Reagent Definition.
From www.youtube.com
Chemistry 12 Lesson 22 Indicators YouTube Indicator Reagent Definition We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. As nouns the. Indicator Reagent Definition.
From www.exit15.com
Taylor Technologies R0004 pH Indicator Reagent, 2 Ounce Indicator Reagent Definition As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know. Indicator Reagent Definition.
From www.slideserve.com
PPT HSC CHEMISTRY CORE TOPIC 2 PowerPoint Presentation, free download ID4556766 Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds. Indicator Reagent Definition.
From ccschemistry.blogspot.com
Cowbridge Chemistry Department Indicators Colours and pH ranges Indicator Reagent Definition They are usually weak acids or bases, but their. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. A reagent which is termed as titrant or titrator is. Indicator Reagent Definition.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their.. Indicator Reagent Definition.
From www.pinterest.com
What are reagent agents reagents in organic chemistry reactions Organic chemistry reactions Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. They are usually weak acids or bases, but their. Chemical indicator, any substance that gives a visible sign, usually by. Indicator Reagent Definition.
From www.youtube.com
Types of Reagents (Detailed Explanation) Organic Chemistry YouTube Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. As. Indicator Reagent Definition.
From www.elephango.com
Describing Chemical Reactions Educational Resources K12 Learning, Chemistry, Science Lesson Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. As nouns the difference between reagent. Indicator Reagent Definition.
From www.slideserve.com
PPT Chapter 16 AcidBase Equilibria PowerPoint Presentation, free download ID2429138 Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a. Indicator Reagent Definition.
From www.youtube.com
Science What Are AcidBase Indicators English YouTube Indicator Reagent Definition We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about. Indicator Reagent Definition.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free download ID4901288 Indicator Reagent Definition They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence. Indicator Reagent Definition.
From testbook.com
Chemical Indicators Learn Definition, Types, Examples and Uses Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as titrant or titrator is prepared on the basis known concentration and volume as a standard solution. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of. Indicator Reagent Definition.
From www.thoughtco.com
What Is a Chemical Indicator? Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change. Indicator Reagent Definition.
From www.youtube.com
What are Indicators and how do we use them? The Chemistry Journey The Fuse School YouTube Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the. Indicator Reagent Definition.
From mobbycorp.com
pH & Reagent Indicators Mobby Business Corporation Indicator Reagent Definition Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the.. Indicator Reagent Definition.
From hxenfosnt.blob.core.windows.net
Chemical Indicators Definition Biology at Lillian Crandell blog Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids. Indicator Reagent Definition.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. A chemical indicator is a substance that undergoes. Indicator Reagent Definition.
From mungfali.com
Examples Of Reactants And Products Indicator Reagent Definition Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. Indicators are substances whose solutions change color due to changes in ph. A reagent. Indicator Reagent Definition.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free download ID4901288 Indicator Reagent Definition They are usually weak acids or bases, but their. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or. Indicator Reagent Definition.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free download ID4901288 Indicator Reagent Definition An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a. Indicator Reagent Definition.
From www.thoughtco.com
What Is a pH Indicator? Definition and Examples Indicator Reagent Definition Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. They are usually. Indicator Reagent Definition.
From www.slideserve.com
PPT Litmus Indicator PowerPoint Presentation, free download ID2790323 Indicator Reagent Definition A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. As nouns the difference between reagent and indicator is that reagent is (chemistry) a compound or mixture of compounds used to. Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical. Indicator Reagent Definition.
From www.chemistrylearner.com
Benedict’s test Definition, Principle, Uses, and Reagent Indicator Reagent Definition They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that. Indicator Reagent Definition.
From pediaa.com
Difference Between Reactant and Reagent Definition, Properties, Examples Indicator Reagent Definition Chemical indicators are those chemical substances in which their presence in chemical reactions helps us to know whether the concentration of a chemical is ideal or not. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A reagent which is termed as. Indicator Reagent Definition.