What Is Water Bond Angle . When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. The answer is the molecular geometry of water would be bent. A bond distance (or bond length) is the. Describe the structure, such as it is, of liquid water. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. Notice there are 4 attachments, or, electron groups surrounding oxygen. What is the molecular geometry and bond angle of water (h 2 o)?
from www.numerade.com
A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. Describe the structure, such as it is, of liquid water. A bond distance (or bond length) is the. What is the molecular geometry and bond angle of water (h 2 o)? When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. The answer is the molecular geometry of water would be bent. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Notice there are 4 attachments, or, electron groups surrounding oxygen. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons.
SOLVED 2. Why is the bond angle in water, H2O, less than the bond
What Is Water Bond Angle A bond distance (or bond length) is the. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? Notice there are 4 attachments, or, electron groups surrounding oxygen. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. The answer is the molecular geometry of water would be bent. A bond distance (or bond length) is the. Describe the structure, such as it is, of liquid water.
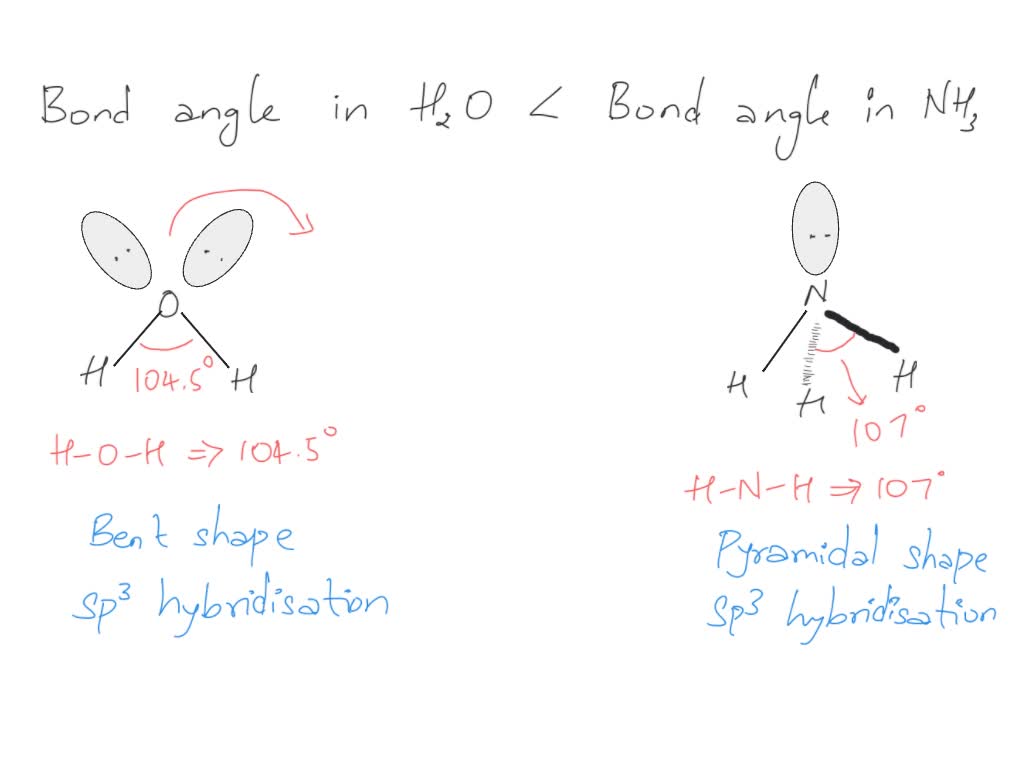
From mungfali.com
Molecular Orbital Diagram Of Water What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. The answer is the molecular geometry of water would be bent. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. A bond distance (or bond length) is the. Explain what is meant by hydrogen bonding and the molecular. What Is Water Bond Angle.
From www.britannica.com
Water Definition, Chemical Formula, Structure, Molecule, & Facts What Is Water Bond Angle The answer is the molecular geometry of water would be bent. What is the molecular geometry and bond angle of water (h 2 o)? A bond distance (or bond length) is the. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. When we predict the ideal bond able. What Is Water Bond Angle.
From socratic.org
What are the strongest bonds in water? Socratic What Is Water Bond Angle When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons.. What Is Water Bond Angle.
From byjus.com
What is the bond angle in a water molecule? What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. A bond distance (or bond length) is the. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Describe the structure, such as it is, of liquid water. When we predict the ideal bond able for h2o (water) we see that it has a. What Is Water Bond Angle.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. What Is Water Bond Angle The answer is the molecular geometry of water would be bent. Notice there are 4 attachments, or, electron groups surrounding oxygen. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. To get the total number of valence electrons for this molecule,. What Is Water Bond Angle.
From www.dreamstime.com
Water Molecule with Bond Angle and Length Stock Vector Illustration What Is Water Bond Angle When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. What is the molecular geometry and bond angle of water (h 2 o)? Notice there are 4 attachments, or, electron groups surrounding oxygen. Describe the structure, such as it is, of liquid. What Is Water Bond Angle.
From www7b.biglobe.ne.jp
Why water H2O bond angle is NOT 90 degree What Is Water Bond Angle The answer is the molecular geometry of water would be bent. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. A bond distance (or bond length) is the. What is the molecular geometry and bond angle of water (h 2 o)? Describe the structure, such as it is,. What Is Water Bond Angle.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is Water Bond Angle What is the molecular geometry and bond angle of water (h 2 o)? Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Notice there are 4 attachments, or, electron groups surrounding oxygen. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons.. What Is Water Bond Angle.
From www.numerade.com
SOLVED 2. Why is the bond angle in water, H2O, less than the bond What Is Water Bond Angle The answer is the molecular geometry of water would be bent. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. Describe the structure, such as it is, of liquid. What Is Water Bond Angle.
From mungfali.com
Lewis Structure Of Water What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond angle is the angle between any two bonds that include a common. What Is Water Bond Angle.
From www.expii.com
Deviation From Ideal Bond Angles — Overview & Examples Expii What Is Water Bond Angle What is the molecular geometry and bond angle of water (h 2 o)? Describe the structure, such as it is, of liquid water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond distance (or bond length) is the. When we predict the ideal bond able for h2o (water) we see that. What Is Water Bond Angle.
From www.science-revision.co.uk
Covalent bonding What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond distance (or bond length) is the. What is the molecular geometry and bond angle of water (h 2 o)? To get the total number of valence electrons for this molecule, we will. What Is Water Bond Angle.
From www.youtube.com
BondAngleWaterlargerthanHydrogensulphide Why Bond Angle in Water is What Is Water Bond Angle Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? The answer is the molecular geometry of water would be bent. When. What Is Water Bond Angle.
From mydigitalkemistry.com
Why the bond angle in ammonia is 107 and that in water is 104 while What Is Water Bond Angle Describe the structure, such as it is, of liquid water. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. The answer is the molecular geometry of water would be. What Is Water Bond Angle.
From wps.prenhall.com
Media Portfolio What Is Water Bond Angle The answer is the molecular geometry of water would be bent. What is the molecular geometry and bond angle of water (h 2 o)? When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. Notice there are 4 attachments, or, electron groups. What Is Water Bond Angle.
From drmchemistrytutor.com
Hydrogen Bonding in water Dr. M. Chemistry Tutor What Is Water Bond Angle A bond distance (or bond length) is the. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. What is the molecular geometry and bond angle of water (h 2 o)? The answer is the molecular geometry of water would be bent. To get the total number of valence electrons. What Is Water Bond Angle.
From owlcation.com
Primary and Secondary Bonds Owlcation What Is Water Bond Angle What is the molecular geometry and bond angle of water (h 2 o)? Notice there are 4 attachments, or, electron groups surrounding oxygen. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. The answer. What Is Water Bond Angle.
From www.chegg.com
Solved The tetrahedral geometry has a bond angle of 109.5° What Is Water Bond Angle Describe the structure, such as it is, of liquid water. The answer is the molecular geometry of water would be bent. Notice there are 4 attachments, or, electron groups surrounding oxygen. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water. What Is Water Bond Angle.
From www.vedantu.com
Why bond angle in water is less than that of ammonia although their What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. A bond distance (or bond length) is the. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. The answer is the molecular geometry of water would be bent. What is the molecular geometry and bond angle of water (h. What Is Water Bond Angle.
From www.youtube.com
Bond Angles for H2O (Ideal and Actual) YouTube What Is Water Bond Angle A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. To get the total number. What Is Water Bond Angle.
From techiescientist.com
H2O Lewis Structure, Molecular Geometry, and Hybridization What Is Water Bond Angle A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? Notice there are 4 attachments, or, electron groups surrounding oxygen. Describe the structure, such as it is, of liquid water. When we predict the ideal bond able for. What Is Water Bond Angle.
From www.chemistrylearner.com
Physical Chemistry Chemistry Learner What Is Water Bond Angle When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. What is the molecular geometry and bond angle of water (h 2 o)? To get the total number of valence electrons for this molecule,. What Is Water Bond Angle.
From www.sciencephoto.com
Bond formation in water molecule Stock Image C028/6477 Science What Is Water Bond Angle The answer is the molecular geometry of water would be bent. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. What is. What Is Water Bond Angle.
From alevelchemistryatthealun.blogspot.com
A Level Chemistry at the Alun Why do you get that 180 bond angle What Is Water Bond Angle What is the molecular geometry and bond angle of water (h 2 o)? When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. The answer is the molecular geometry of water would be bent. Describe the structure, such as it is, of liquid water. Explain what is meant by hydrogen. What Is Water Bond Angle.
From necosmallsite.blogspot.com
Is Water A Compound Neco What Is Water Bond Angle The answer is the molecular geometry of water would be bent. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond angle is the angle between any. What Is Water Bond Angle.
From dinosenglish.edu.vn
Arriba 90+ Foto Estructura De Lewis Del Agua H2o Cena Hermosa What Is Water Bond Angle Notice there are 4 attachments, or, electron groups surrounding oxygen. The answer is the molecular geometry of water would be bent. What is the molecular geometry and bond angle of water (h 2 o)? A bond distance (or bond length) is the. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen. What Is Water Bond Angle.
From techiescientist.com
H2O Lewis Structure, Molecular Geometry, and Hybridization What Is Water Bond Angle A bond distance (or bond length) is the. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. The answer is the molecular geometry of water would be bent. What is. What Is Water Bond Angle.
From mungfali.com
Hydrogen Bonding Diagram What Is Water Bond Angle The answer is the molecular geometry of water would be bent. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? Describe. What Is Water Bond Angle.
From www.youtube.com
bond angle of water molecule english medium venkipedia YouTube What Is Water Bond Angle A bond distance (or bond length) is the. The answer is the molecular geometry of water would be bent. Notice there are 4 attachments, or, electron groups surrounding oxygen. What is the molecular geometry and bond angle of water (h 2 o)? Describe the structure, such as it is, of liquid water. A bond angle is the angle between any. What Is Water Bond Angle.
From www7b.biglobe.ne.jp
Why water H2O bond angle is NOT 90 degree What Is Water Bond Angle Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. What is the molecular geometry and bond angle of water (h 2 o)? Describe the structure,. What Is Water Bond Angle.
From errantscience.com
ErrantScience Water surface tension What Is Water Bond Angle Describe the structure, such as it is, of liquid water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond distance (or bond length) is the. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. A bond angle is the. What Is Water Bond Angle.
From www.youtube.com
Water Molecular Geometry and Bond Angles YouTube What Is Water Bond Angle A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Notice there are 4 attachments, or, electron groups surrounding oxygen. The answer. What Is Water Bond Angle.
From www.toppr.com
The H O H bond angle in the water molecule is 105^o , the H O What Is Water Bond Angle What is the molecular geometry and bond angle of water (h 2 o)? Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. When we predict the ideal bond able for h2o (water). What Is Water Bond Angle.
From www7b.biglobe.ne.jp
Why water H2O bond angle is NOT 90 degree What Is Water Bond Angle Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. When we predict the ideal bond able for h2o (water) we see that it has a bent molecular geometry and. A bond distance (or bond length) is the. To get the total number of valence electrons for this molecule, we will add up hydrogen. What Is Water Bond Angle.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure What Is Water Bond Angle Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. What is the molecular geometry and bond angle of water (h 2 o)? Describe the structure, such as it is, of liquid water. Notice there. What Is Water Bond Angle.