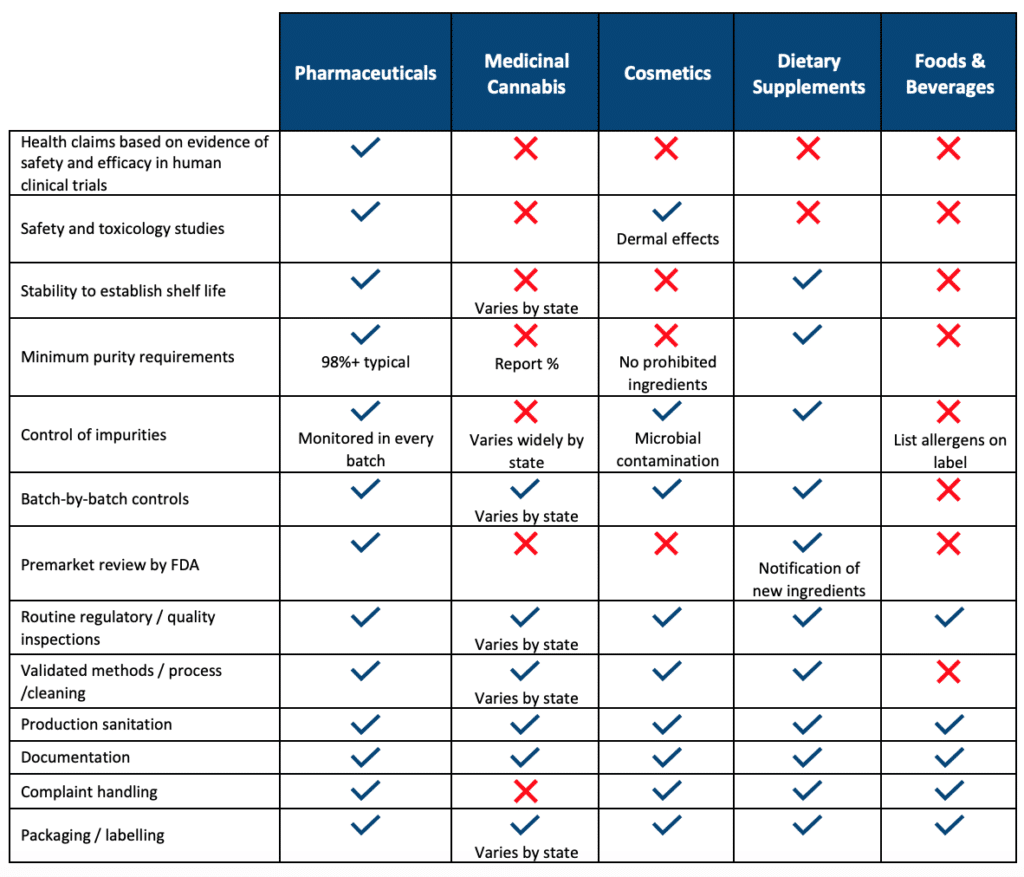
Gmp Laboratory Requirements . Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide.
from www.inmedpharma.com
Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp.
What are the GMP manufacturing standards for different products?
Gmp Laboratory Requirements The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational.
From www.scilife.io
What are the 5 Main Components of GMP? Full definition Scilife Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred. Gmp Laboratory Requirements.
From gmp.com.vn
Comparison of the requirements of EU GMP guidelines versus WHO GMP Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From solutionpharmacy.in
GMP Requirements Solution Parmacy Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. Gmp Laboratory Requirements.
From www.scribd.com
GMP Requirements For Certificates of Analysis PDF Gmp Laboratory Requirements The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as. Gmp Laboratory Requirements.
From www.slideserve.com
PPT Aspects of GMP PowerPoint Presentation, free download ID2919842 Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect. Gmp Laboratory Requirements.
From www.compliancequest.com
What is GMP Quality? GMP Standards and Regulations Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. Gmp Laboratory Requirements.
From quallpharma.blogspot.com
Quallpharma consultancy The GMP Systems (General Requirement) Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Questions and answers on current good manufacturing practice requirements | laboratory controls Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp). Gmp Laboratory Requirements.
From www.slideserve.com
PPT Good Manufacturing Practices (GMP), Good Laboratory Practices Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Questions and answers on current good manufacturing practice requirements | laboratory controls Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp). Gmp Laboratory Requirements.
From www.scilife.io
5 Essential Components of GMP A Comprehensive Guide Gmp Laboratory Requirements Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ),. Gmp Laboratory Requirements.
From www.butterworth-labs.co.uk
GMP Butterworth Laboratories Gmp Laboratory Requirements Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Questions and answers on current good manufacturing practice requirements | laboratory controls Directives 2001/82/ec and 2001/83/ec, as amended. Gmp Laboratory Requirements.
From www.pharmaaccess.net
The GMP Framework Pharmaaccess Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices. Gmp Laboratory Requirements.
From www.slideshare.net
GMP Requirements in laboratories 2016 at Mumbai Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From www.scribd.com
GMP Checklist Sterilization (Microbiology) Filtration Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp). Gmp Laboratory Requirements.
From labcollector.com
Regulations Compliance LabCollector Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From www.presentationeze.com
GMP Training Information and Training on GMP requirements.PresentationEZE Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICE FOR BIOPROCESS ENGINEERING (ERT 421 Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect. Gmp Laboratory Requirements.
From akap.ca
GMP Good Manufacturing Practices (New Method2021) Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good manufacturing practices (gmp, also. Gmp Laboratory Requirements.
From www.europeanpharmaceuticalreview.com
Balancing regulations for weighing in a GMP quality control laboratory Gmp Laboratory Requirements The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Questions and answers on current good manufacturing practice requirements | laboratory controls Good manufacturing practices (gmp, also. Gmp Laboratory Requirements.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of. Gmp Laboratory Requirements.
From dokumen.tips
(PDF) Regulatory Basics for Facility Design (WHO GMP) Biosafety Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice. Gmp Laboratory Requirements.
From www.htgroup.de
GMP laboratories in hospitals HT GROUP Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system. Gmp Laboratory Requirements.
From www.pharmaspecialists.com
GMP Guidelines for Pharmaceutical Industry Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. The principles of good laboratory practice. Gmp Laboratory Requirements.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Directives 2001/82/ec and 2001/83/ec, as amended. Gmp Laboratory Requirements.
From oms-technology.com
Pharmaceutical GMP Cleanroom Biosafety laboratory design Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system. Gmp Laboratory Requirements.
From www.slideserve.com
PPT Aspects of GMP PowerPoint Presentation, free download ID2919842 Gmp Laboratory Requirements Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice (glp). Gmp Laboratory Requirements.
From www.vecteezy.com
GMP Good Manufacturing Practice 6 heading of infographic template with Gmp Laboratory Requirements Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection, a gmp. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Laboratory Requirements The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect. Gmp Laboratory Requirements.
From www.vrogue.co
Pharmaceutical Quality Control Framework With Gmp Com vrogue.co Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Gmp Laboratory Requirements.
From operonstrategist.com
GMP Certificate for Medical Devices (Standards and Requirements Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Guidelines for good manufacturing practices (1). Gmp Laboratory Requirements.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICE FOR BIOPROCESS ENGINEERING (ERT 421 Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Questions and answers on current good manufacturing practice requirements | laboratory controls The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as. Gmp Laboratory Requirements.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Questions and answers on current good manufacturing practice requirements | laboratory controls Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The principles of good laboratory practice (glp). Gmp Laboratory Requirements.
From www.vietfil.com
What is GMP standard in pharmaceutical manufacturing Gmp Laboratory Requirements Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection,. Gmp Laboratory Requirements.
From www.inmedpharma.com
What are the GMP manufacturing standards for different products? Gmp Laboratory Requirements Questions and answers on current good manufacturing practice requirements | laboratory controls Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Directives 2001/82/ec and 2001/83/ec, as. Gmp Laboratory Requirements.
From allevents.in
GMP requirements for quality control and contract laboratories 2017 at Gmp Laboratory Requirements Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection,. Gmp Laboratory Requirements.
From ndgcs.com
Principles and Compliance Requirements of GMP/GLP for Quality Control Gmp Laboratory Requirements Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Guidelines for good manufacturing practices (1) and with the requirements of the international standard iso/iec 17025:2005 ( 2 ), and provide. Directives 2001/82/ec and 2001/83/ec, as amended state that after every gmp inspection, and within 90 days of the inspection,. Gmp Laboratory Requirements.