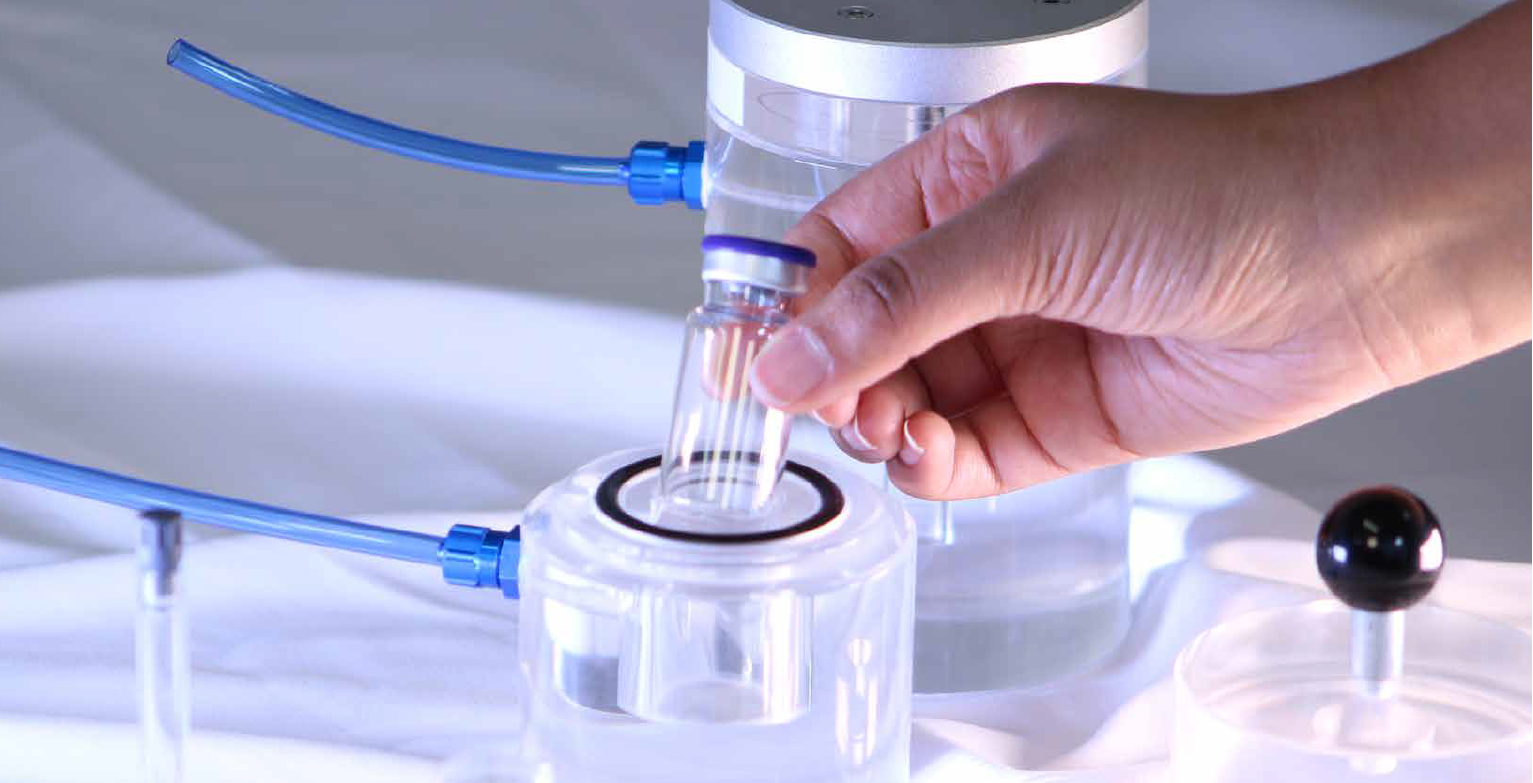
Ccit Vacuum Decay Method . Container closure integrity control strategy development. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about the three common methods: Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Product lifecycle and cci testing.
from www.pti-ccit.com
Product lifecycle and cci testing. Learn about the three common methods: Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development.
Vacuum Decay, The Ultimate Solution
Ccit Vacuum Decay Method The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal.
From blog.sepha.com
Vacuum Decay Testing of Parenterals Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about the three common methods: Container closure integrity control strategy development. The standard vacuum decay leak test. Ccit Vacuum Decay Method.
From www.bbsautomation.com
Various Testing Methods BBS Automation Ccit Vacuum Decay Method The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing. Container closure integrity control strategy development. Learn about the three common methods: Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination.. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay Technology for Ophthalmic Product Package Inspection Ccit Vacuum Decay Method Learn about the three common methods: Container closure integrity control strategy development. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination.. Ccit Vacuum Decay Method.
From ccit-pharma-showroom.pfeiffer-vacuum.com
to our CCITShowroom Pfeiffer Vacuum Showroom Ccit Vacuum Decay Method Product lifecycle and cci testing. Container closure integrity control strategy development. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about the three common methods: Learn about. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Medical Device Package Inspection using Vacuum Decay Technology Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to. Ccit Vacuum Decay Method.
From www.pti-ccit.com
How is Vacuum Decay Technology an Ideal Nutrition Package Inspection Ccit Vacuum Decay Method Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Ccit testing is a quality check. Ccit Vacuum Decay Method.
From www.ccit.com
Everything You Need to Know About Vacuum Decay Technology Ccit Vacuum Decay Method The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development. Learn about the three common methods: Ccit testing is a quality. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay, The Ultimate Solution Ccit Vacuum Decay Method Product lifecycle and cci testing. Container closure integrity control strategy development. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about the three common methods:. Ccit Vacuum Decay Method.
From sepha.com
Vacuum Decay Test Nondestructive & Deterministic CCIT Ccit Vacuum Decay Method Product lifecycle and cci testing. Container closure integrity control strategy development. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a.. Ccit Vacuum Decay Method.
From www.semanticscholar.org
Table VIII from Vacuum Decay Container Closure Integrity Leak Test Ccit Vacuum Decay Method Learn about the three common methods: Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Product lifecycle and cci testing. Learn about. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay, The Ultimate Solution Ccit Vacuum Decay Method Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Container closure integrity control strategy development. Learn about the three common methods: Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test. Ccit Vacuum Decay Method.
From www.researchgate.net
A schematic picture showing a vacuum decay catalyzed by a static and Ccit Vacuum Decay Method Product lifecycle and cci testing. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about container closure integrity testing (ccit), a. Ccit Vacuum Decay Method.
From www.ccit.com
Prefilled Syringes Leak Detection with Vacuum Decay Vs MicroCurrent Ccit Vacuum Decay Method Learn about the three common methods: Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about container closure integrity testing (ccit), a critical evaluation of container closure. Ccit Vacuum Decay Method.
From www.pharmaceutical-networking.com
Bonfiglioli Vacuum Decay Leak Detection for Prefilled Syringes Ccit Vacuum Decay Method Product lifecycle and cci testing. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about container closure integrity testing (ccit), a. Ccit Vacuum Decay Method.
From www.ccit.com
Evaluating Pharmaceutical Package Sterility with Vacuum Decay Technology Ccit Vacuum Decay Method Learn about the three common methods: Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The. Ccit Vacuum Decay Method.
From blog.sepha.com
USP 1207 Opening the CCIT toolbox Ccit Vacuum Decay Method Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development. Learn about the three common methods: Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The. Ccit Vacuum Decay Method.
From sepha.com
Vacuum decay method Nondestructive CCIT Sepha MultiQ Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Container closure integrity control strategy development. Learn about the three common methods: Product lifecycle and cci testing. The. Ccit Vacuum Decay Method.
From www.ccit.com
Why use Vacuum Decay Technology for Ophthalmic Product Package Inspection Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Product lifecycle and cci testing.. Ccit Vacuum Decay Method.
From www.youtube.com
Vacuum Decay Technology Container Closure Integrity Testing VeriPac Ccit Vacuum Decay Method Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Learn about the three common methods:. Ccit Vacuum Decay Method.
From www.ascinstrument.com
ASC Instrument CCIT Test Equipment Vacuum decay Pressure decay Ccit Vacuum Decay Method Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Product lifecycle and cci testing. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method. Ccit Vacuum Decay Method.
From www.ccit.com
Vacuum Decay TechnologyA Novel Solution for Nutritional Packaging Ccit Vacuum Decay Method Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about the three common methods: The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing. Learn about. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay Technology An Overview of Applications Ccit Vacuum Decay Method Learn about the three common methods: Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing. Learn about. Ccit Vacuum Decay Method.
From www.sanatron.com
Anatomy of the Pressure Decay, Vacuum Decay, and Force Decay Curve Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Product lifecycle and cci testing. Learn about the three common methods:. Ccit Vacuum Decay Method.
From www.wilco.com
Nondestructive leak testing with differential pressure technology Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Product lifecycle and cci testing. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Ccit testing is a quality check. Ccit Vacuum Decay Method.
From www.ccit.com
Vacuum Decay Technology Raising Standards for Pharmaceutical Package Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The standard vacuum decay leak test method (astm f2338), developed using pti's. Ccit Vacuum Decay Method.
From www.youtube.com
Vacuum Decay Test of Vials, Ampoules, Bottles, PFS, BFS Down to 5µm Ccit Vacuum Decay Method The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: Ccit testing is a quality. Ccit Vacuum Decay Method.
From exopyaqfs.blob.core.windows.net
How Vacuum Decay at Amanda Emery blog Ccit Vacuum Decay Method Container closure integrity control strategy development. Learn about the three common methods: Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Product lifecycle and cci testing.. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay Technology for Pharmaceutical Package Inspection Ccit Vacuum Decay Method Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Product lifecycle and cci testing. Learn about the three common methods: The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Learn about. Ccit Vacuum Decay Method.
From www.ccit.com
Applications of Vacuum Decay Technology Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: Container closure integrity control strategy development. Product lifecycle and cci testing. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. The. Ccit Vacuum Decay Method.
From www.semanticscholar.org
Figure 1 from Vacuum Decay Container Closure Integrity Leak Test Method Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Learn about the three common methods: Product lifecycle and cci testing. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Container closure integrity control strategy development. The. Ccit Vacuum Decay Method.
From www.ccit.com
CCI Testing of Intravenous Bags Using Vacuum Decay Technology Ccit Vacuum Decay Method Learn about the three common methods: The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Product lifecycle and cci testing. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about. Ccit Vacuum Decay Method.
From www.youtube.com
Container Closure Integrity Testing CCIT Parenterals Vacuum Ccit Vacuum Decay Method Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to. Ccit Vacuum Decay Method.
From www.youtube.com
Vacuum Decay Closure Integrity Testing Method Bonfiglioli Engineering Ccit Vacuum Decay Method Learn about the three common methods: Product lifecycle and cci testing. The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Container closure integrity control strategy development. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination.. Ccit Vacuum Decay Method.
From dev.ccit.com
Vacuum Decay Technology for Quality Control Assurance of Parenteral Ccit Vacuum Decay Method The standard vacuum decay leak test method (astm f2338), developed using pti's veripac instruments, is recognized by the fda as a. Learn about the three common methods: Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Ccit testing is a quality check for pharmaceuticals that evaluates the. Ccit Vacuum Decay Method.
From www.pti-ccit.com
Vacuum Decay Testing for CGMs Enhancing Package Integrity and Patient Ccit Vacuum Decay Method Ccit testing is a quality check for pharmaceuticals that evaluates the container closure and its ability to maintain a seal. Learn about container closure integrity testing (ccit), a critical evaluation of container closure systems’ ability to maintain a sterile barrier against contamination. Container closure integrity control strategy development. Product lifecycle and cci testing. Learn about the three common methods: The. Ccit Vacuum Decay Method.