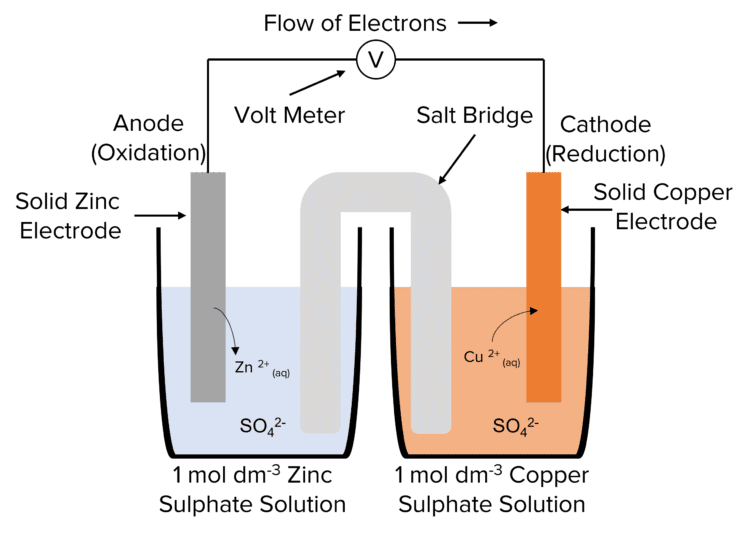
Electrochemical Cells In Everyday Life . Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. What is an electrochemical cell? In principle, any galvanic cell could be used as a battery. An ideal battery would never run down,. We will look at three electrolytic cells and the quantitative aspects of electrolysis. There are two types of electrochemical cells. In molten sodium chloride, the ions are free to migrate to. What is a half cell? A battery is an electrochemical cell or series of cells that produces an electric current. All sorts of batteries, from those used to power a flashlight to a calculator to an. Electrochemical cells are essential devices that convert chemical energy into electrical energy. What is a salt bridge? What are the types of electrochemical cells? Electrochemistry has many common applications in everyday life.
from mmerevise.co.uk
An ideal battery would never run down,. A battery is an electrochemical cell or series of cells that produces an electric current. What are the types of electrochemical cells? In molten sodium chloride, the ions are free to migrate to. Electrochemistry has many common applications in everyday life. Electrochemical cells are essential devices that convert chemical energy into electrical energy. What is an electrochemical cell? In principle, any galvanic cell could be used as a battery. What is a salt bridge? We will look at three electrolytic cells and the quantitative aspects of electrolysis.
Electrochemical Cells MME
Electrochemical Cells In Everyday Life Electrochemistry has many common applications in everyday life. Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. What is an electrochemical cell? Electrochemical cells are essential devices that convert chemical energy into electrical energy. An ideal battery would never run down,. What are the types of electrochemical cells? All sorts of batteries, from those used to power a flashlight to a calculator to an. Electrochemistry has many common applications in everyday life. What is a half cell? What is a salt bridge? We will look at three electrolytic cells and the quantitative aspects of electrolysis. A battery is an electrochemical cell or series of cells that produces an electric current. In molten sodium chloride, the ions are free to migrate to. In principle, any galvanic cell could be used as a battery. There are two types of electrochemical cells.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cells In Everyday Life A battery is an electrochemical cell or series of cells that produces an electric current. All sorts of batteries, from those used to power a flashlight to a calculator to an. What are the types of electrochemical cells? In molten sodium chloride, the ions are free to migrate to. What is a half cell? What is a salt bridge? We. Electrochemical Cells In Everyday Life.
From hxervxenb.blob.core.windows.net
Examples Of Electrochemistry In Everyday Life at Andrew Price blog Electrochemical Cells In Everyday Life An ideal battery would never run down,. Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. What is a salt bridge? In principle, any galvanic cell could be used as a battery. A battery is an electrochemical cell or series of cells that produces an electric current.. Electrochemical Cells In Everyday Life.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemical Cells In Everyday Life An ideal battery would never run down,. In molten sodium chloride, the ions are free to migrate to. Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. Electrochemical cells are essential devices that convert chemical energy into electrical energy. In principle, any galvanic cell could be used. Electrochemical Cells In Everyday Life.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy Electrochemical Cells In Everyday Life What is a half cell? What are the types of electrochemical cells? An ideal battery would never run down,. A battery is an electrochemical cell or series of cells that produces an electric current. What is a salt bridge? What is an electrochemical cell? All sorts of batteries, from those used to power a flashlight to a calculator to an.. Electrochemical Cells In Everyday Life.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemical Cells In Everyday Life What is a half cell? A battery is an electrochemical cell or series of cells that produces an electric current. An ideal battery would never run down,. There are two types of electrochemical cells. All sorts of batteries, from those used to power a flashlight to a calculator to an. Electrochemical cells in which spontaneous redox reactions take place (galvanic. Electrochemical Cells In Everyday Life.
From www.haikudeck.com
Electrochemistry by blisaac Electrochemical Cells In Everyday Life What is an electrochemical cell? Electrochemistry has many common applications in everyday life. A battery is an electrochemical cell or series of cells that produces an electric current. An ideal battery would never run down,. In molten sodium chloride, the ions are free to migrate to. We will look at three electrolytic cells and the quantitative aspects of electrolysis. What. Electrochemical Cells In Everyday Life.
From 2012books.lardbucket.org
Describing Electrochemical Cells Electrochemical Cells In Everyday Life Electrochemistry has many common applications in everyday life. Electrochemical cells are essential devices that convert chemical energy into electrical energy. What is a salt bridge? What are the types of electrochemical cells? Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. A battery is an electrochemical cell. Electrochemical Cells In Everyday Life.
From www.firsthope.co.in
Electrochemical cell Electrochemical Cells In Everyday Life What are the types of electrochemical cells? Electrochemistry has many common applications in everyday life. All sorts of batteries, from those used to power a flashlight to a calculator to an. In molten sodium chloride, the ions are free to migrate to. What is a half cell? Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been. Electrochemical Cells In Everyday Life.
From en.ppt-online.org
Electrochemistry online presentation Electrochemical Cells In Everyday Life Electrochemical cells are essential devices that convert chemical energy into electrical energy. A battery is an electrochemical cell or series of cells that produces an electric current. Electrochemistry has many common applications in everyday life. In molten sodium chloride, the ions are free to migrate to. There are two types of electrochemical cells. What is a salt bridge? What are. Electrochemical Cells In Everyday Life.
From aryan-blogduke.blogspot.com
Daniell Cell Class 12 Electrochemical Cells In Everyday Life An ideal battery would never run down,. What is an electrochemical cell? All sorts of batteries, from those used to power a flashlight to a calculator to an. There are two types of electrochemical cells. What is a half cell? What is a salt bridge? In principle, any galvanic cell could be used as a battery. Electrochemical cells in which. Electrochemical Cells In Everyday Life.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrochemical Cells In Everyday Life What is an electrochemical cell? What is a salt bridge? What is a half cell? All sorts of batteries, from those used to power a flashlight to a calculator to an. There are two types of electrochemical cells. In principle, any galvanic cell could be used as a battery. Electrochemistry has many common applications in everyday life. We will look. Electrochemical Cells In Everyday Life.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6568172 Electrochemical Cells In Everyday Life Electrochemical cells are essential devices that convert chemical energy into electrical energy. An ideal battery would never run down,. What is an electrochemical cell? All sorts of batteries, from those used to power a flashlight to a calculator to an. A battery is an electrochemical cell or series of cells that produces an electric current. There are two types of. Electrochemical Cells In Everyday Life.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemical Cells In Everyday Life Electrochemical cells are essential devices that convert chemical energy into electrical energy. An ideal battery would never run down,. What is an electrochemical cell? Electrochemistry has many common applications in everyday life. In molten sodium chloride, the ions are free to migrate to. There are two types of electrochemical cells. All sorts of batteries, from those used to power a. Electrochemical Cells In Everyday Life.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for Electrochemical Cells In Everyday Life What is a half cell? What is a salt bridge? In molten sodium chloride, the ions are free to migrate to. What is an electrochemical cell? We will look at three electrolytic cells and the quantitative aspects of electrolysis. An ideal battery would never run down,. What are the types of electrochemical cells? A battery is an electrochemical cell or. Electrochemical Cells In Everyday Life.
From www.vrogue.co
Examples Of Galvanic Cells In Everyday Life Studiousg vrogue.co Electrochemical Cells In Everyday Life In molten sodium chloride, the ions are free to migrate to. Electrochemical cells are essential devices that convert chemical energy into electrical energy. We will look at three electrolytic cells and the quantitative aspects of electrolysis. Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. An ideal. Electrochemical Cells In Everyday Life.
From chem.libretexts.org
Commercial Galvanic Cells Chemistry LibreTexts Electrochemical Cells In Everyday Life Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. There are two types of electrochemical cells. In molten sodium chloride, the ions are free to migrate to. An ideal battery would never run down,. Electrochemistry has many common applications in everyday life. What is an electrochemical cell?. Electrochemical Cells In Everyday Life.
From www.youtube.com
ELECTROCHEMICAL CELL HINDI EXPLANATION ELECTROCHEMISTRY 12TH Electrochemical Cells In Everyday Life Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. What is a half cell? In principle, any galvanic cell could be used as a battery. There are two types of electrochemical cells. What is an electrochemical cell? All sorts of batteries, from those used to power a. Electrochemical Cells In Everyday Life.
From studylib.net
Introduction to Electrochemistry Electrochemical Cells In Everyday Life All sorts of batteries, from those used to power a flashlight to a calculator to an. Electrochemical cells are essential devices that convert chemical energy into electrical energy. A battery is an electrochemical cell or series of cells that produces an electric current. In molten sodium chloride, the ions are free to migrate to. We will look at three electrolytic. Electrochemical Cells In Everyday Life.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation ID3838900 Electrochemical Cells In Everyday Life What is a half cell? Electrochemical cells are essential devices that convert chemical energy into electrical energy. In molten sodium chloride, the ions are free to migrate to. What is a salt bridge? All sorts of batteries, from those used to power a flashlight to a calculator to an. An ideal battery would never run down,. There are two types. Electrochemical Cells In Everyday Life.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Electrochemical Cells In Everyday Life An ideal battery would never run down,. What is a half cell? What is a salt bridge? In principle, any galvanic cell could be used as a battery. A battery is an electrochemical cell or series of cells that produces an electric current. Electrochemical cells are essential devices that convert chemical energy into electrical energy. There are two types of. Electrochemical Cells In Everyday Life.
From 2012books.lardbucket.org
Describing Electrochemical Cells Electrochemical Cells In Everyday Life There are two types of electrochemical cells. Electrochemistry has many common applications in everyday life. In principle, any galvanic cell could be used as a battery. Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. All sorts of batteries, from those used to power a flashlight to. Electrochemical Cells In Everyday Life.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical Electrochemical Cells In Everyday Life Electrochemistry has many common applications in everyday life. A battery is an electrochemical cell or series of cells that produces an electric current. There are two types of electrochemical cells. Electrochemical cells are essential devices that convert chemical energy into electrical energy. What are the types of electrochemical cells? What is an electrochemical cell? In principle, any galvanic cell could. Electrochemical Cells In Everyday Life.
From www.slideserve.com
PPT Electrolytic Cell PowerPoint Presentation, free download ID2809508 Electrochemical Cells In Everyday Life There are two types of electrochemical cells. In molten sodium chloride, the ions are free to migrate to. A battery is an electrochemical cell or series of cells that produces an electric current. What are the types of electrochemical cells? Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in. Electrochemical Cells In Everyday Life.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Electrochemical Cells In Everyday Life All sorts of batteries, from those used to power a flashlight to a calculator to an. A battery is an electrochemical cell or series of cells that produces an electric current. An ideal battery would never run down,. There are two types of electrochemical cells. Electrochemical cells are essential devices that convert chemical energy into electrical energy. In principle, any. Electrochemical Cells In Everyday Life.
From www.researchgate.net
(PDF) Electrochemical Cells An Introduction Electrochemical Cells In Everyday Life There are two types of electrochemical cells. An ideal battery would never run down,. In principle, any galvanic cell could be used as a battery. A battery is an electrochemical cell or series of cells that produces an electric current. Electrochemistry has many common applications in everyday life. In molten sodium chloride, the ions are free to migrate to. What. Electrochemical Cells In Everyday Life.
From facts.net
15 Surprising Facts About Electrochemical Cell Electrochemical Cells In Everyday Life There are two types of electrochemical cells. What is an electrochemical cell? We will look at three electrolytic cells and the quantitative aspects of electrolysis. Electrochemical cells are essential devices that convert chemical energy into electrical energy. What are the types of electrochemical cells? What is a salt bridge? All sorts of batteries, from those used to power a flashlight. Electrochemical Cells In Everyday Life.
From hxervxenb.blob.core.windows.net
Examples Of Electrochemistry In Everyday Life at Andrew Price blog Electrochemical Cells In Everyday Life What are the types of electrochemical cells? A battery is an electrochemical cell or series of cells that produces an electric current. What is a half cell? What is an electrochemical cell? Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. Electrochemistry has many common applications in. Electrochemical Cells In Everyday Life.
From www.thoughtco.com
Electrochemical Cell EMF Example Problem Electrochemical Cells In Everyday Life Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. There are two types of electrochemical cells. What is an electrochemical cell? We will look at three electrolytic cells and the quantitative aspects of electrolysis. What is a half cell? An ideal battery would never run down,. What. Electrochemical Cells In Everyday Life.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy Electrochemical Cells In Everyday Life Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. An ideal battery would never run down,. What is an electrochemical cell? All sorts of batteries, from those used to power a flashlight to a calculator to an. In principle, any galvanic cell could be used as a. Electrochemical Cells In Everyday Life.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Electrochemical Cells In Everyday Life There are two types of electrochemical cells. An ideal battery would never run down,. What are the types of electrochemical cells? What is a half cell? What is a salt bridge? Electrochemical cells are essential devices that convert chemical energy into electrical energy. A battery is an electrochemical cell or series of cells that produces an electric current. Electrochemical cells. Electrochemical Cells In Everyday Life.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemical Cells In Everyday Life What is a half cell? All sorts of batteries, from those used to power a flashlight to a calculator to an. In molten sodium chloride, the ions are free to migrate to. A battery is an electrochemical cell or series of cells that produces an electric current. We will look at three electrolytic cells and the quantitative aspects of electrolysis.. Electrochemical Cells In Everyday Life.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical Cells In Everyday Life Electrochemical cells are essential devices that convert chemical energy into electrical energy. An ideal battery would never run down,. What is a half cell? What are the types of electrochemical cells? Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. In principle, any galvanic cell could be. Electrochemical Cells In Everyday Life.
From studylib.net
3.ElectrochemicalCells Electrochemical Cells In Everyday Life Electrochemistry has many common applications in everyday life. A battery is an electrochemical cell or series of cells that produces an electric current. What are the types of electrochemical cells? Electrochemical cells are essential devices that convert chemical energy into electrical energy. We will look at three electrolytic cells and the quantitative aspects of electrolysis. What is a salt bridge?. Electrochemical Cells In Everyday Life.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Electrochemical Cells In Everyday Life In molten sodium chloride, the ions are free to migrate to. A battery is an electrochemical cell or series of cells that produces an electric current. What is an electrochemical cell? What is a half cell? We will look at three electrolytic cells and the quantitative aspects of electrolysis. In principle, any galvanic cell could be used as a battery.. Electrochemical Cells In Everyday Life.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemical Cells In Everyday Life There are two types of electrochemical cells. In principle, any galvanic cell could be used as a battery. Electrochemical cells are essential devices that convert chemical energy into electrical energy. We will look at three electrolytic cells and the quantitative aspects of electrolysis. An ideal battery would never run down,. What is a half cell? All sorts of batteries, from. Electrochemical Cells In Everyday Life.