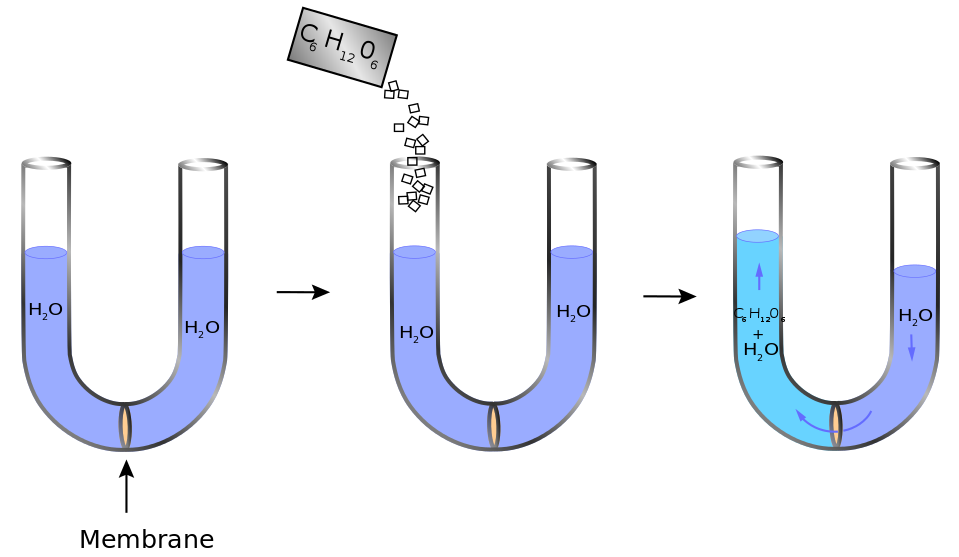
Osmotic Pressure Vs Osmolality . In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Concept of osmolality and milliequivalent. It is defined as the molarity. Both osmolarity and osmolality are defined in terms of osmoles. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic.
from pediaa.com
Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Both osmolarity and osmolality are defined in terms of osmoles. Concept of osmolality and milliequivalent. It is defined as the molarity. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are.
Difference Between Osmolarity and Osmolality Definition, Explanation
Osmotic Pressure Vs Osmolality Concept of osmolality and milliequivalent. Concept of osmolality and milliequivalent. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. It is defined as the molarity. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Both osmolarity and osmolality are defined in terms of osmoles. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns.
From www.adda247.com
Osmotic Pressure formula in Biology Class 12 Osmotic Pressure Vs Osmolality Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Osmotic Pressure // Solutions[Colligative properties] pt1 YouTube Osmotic Pressure Vs Osmolality Both osmolarity and osmolality are defined in terms of osmoles. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Osmolarity (osmol) is a way of reporting the total number of particles in. Osmotic Pressure Vs Osmolality.
From www.pinterest.com
Difference Between Osmolarity and Tonicity infographic Biology facts Osmotic Pressure Vs Osmolality Concept of osmolality and milliequivalent. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Thus, osmolality is a measure of the osmotic pressure exerted by a. Osmotic Pressure Vs Osmolality.
From pediaa.com
Difference Between Hydrostatic and Osmotic Pressure Definition Osmotic Pressure Vs Osmolality Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Guyton chapter 4 Osmosis summary osmotic pressure Osmolality Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Concept of osmolality and milliequivalent. An osmole is a unit of measurement that describes the. Osmotic Pressure Vs Osmolality.
From basicmedicalkey.com
Body Fluids and Water Balance Basicmedical Key Osmotic Pressure Vs Osmolality An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Concept of osmolality and milliequivalent. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in. Osmotic Pressure Vs Osmolality.
From handwiki.org
BiologyOncotic pressure HandWiki Osmotic Pressure Vs Osmolality Both osmolarity and osmolality are defined in terms of osmoles. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the. Osmotic Pressure Vs Osmolality.
From differencebtw.com
Osmotic Pressure vs. Oncotic Pressure Know the Difference Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. It is defined as the molarity. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Concept of osmolality and milliequivalent. Both osmolarity and osmolality are defined in terms of osmoles. The osmotic. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series Osmotic Pressure Vs Osmolality The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference. Osmotic Pressure Vs Osmolality.
From medicinespecifics.com
Nephrology Medicine Specifics Osmotic Pressure Vs Osmolality The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Both osmolarity and osmolality are defined in terms of osmoles. In order to calculate osmotic pressure, it. Osmotic Pressure Vs Osmolality.
From www.thoughtco.com
Osmotic Pressure and Tonicity Osmotic Pressure Vs Osmolality Both osmolarity and osmolality are defined in terms of osmoles. It is defined as the molarity. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Thus, osmolality is a measure of the. Osmotic Pressure Vs Osmolality.
From www.researchgate.net
Osmotic agents with their osmotic pressure. 4446 Download Table Osmotic Pressure Vs Osmolality In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Concept of osmolality and milliequivalent. Both osmolarity and osmolality are defined in terms of osmoles. An osmole is a unit of measurement that describes the number of moles of. Osmotic Pressure Vs Osmolality.
From pediaa.com
Difference Between Osmotic Pressure and Oncotic Pressure Osmotic Pressure Vs Osmolality The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. An osmole is a unit of measurement that describes the number of moles of a compound that. Osmotic Pressure Vs Osmolality.
From www.biologyonline.com
Osmotic pressure Definition and Examples Biology Online Dictionary Osmotic Pressure Vs Osmolality In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Both osmolarity and osmolality are defined in terms of osmoles. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the. Osmotic Pressure Vs Osmolality.
From www.youtube.com
44Hemodynamic Disordersreduced plasma osmotic pressure, osmosis Osmotic Pressure Vs Osmolality Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Both osmolarity and osmolality are defined in terms of osmoles. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. An osmole is a. Osmotic Pressure Vs Osmolality.
From inspiritvr.com
Osmotic Pressure Study Guide Inspirit Osmotic Pressure Vs Osmolality The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference. Osmotic Pressure Vs Osmolality.
From www.slideserve.com
PPT IV Therapy PowerPoint Presentation, free download ID9628818 Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Concept of osmolality and milliequivalent. The standard definition. Osmotic Pressure Vs Osmolality.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Osmotic Pressure Vs Osmolality The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Concept of osmolality and milliequivalent. It is defined as the molarity. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolarity (osmol) is a. Osmotic Pressure Vs Osmolality.
From www.researchgate.net
Schematic representation of fluid homeostatic relationships between Osmotic Pressure Vs Osmolality Concept of osmolality and milliequivalent. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable. Osmotic Pressure Vs Osmolality.
From www.slideserve.com
PPT Importance of Osmosis and Osmotic Pressure PowerPoint Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Concept of osmolality and milliequivalent. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case. Osmotic Pressure Vs Osmolality.
From www.slideserve.com
PPT The urinary system homeostasis and temperature control PowerPoint Osmotic Pressure Vs Osmolality The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. It is defined as the molarity. An osmole is a unit of measurement that describes the number. Osmotic Pressure Vs Osmolality.
From www.vrogue.co
What Is Osmosis vrogue.co Osmotic Pressure Vs Osmolality An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. It is defined as the molarity. The standard definition of tonicity usually incorporates some mention of osmotic. Osmotic Pressure Vs Osmolality.
From www.geeksforgeeks.org
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs Osmotic Pressure Vs Osmolality Concept of osmolality and milliequivalent. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Effect of Osmotic Pressure Lab YouTube Osmotic Pressure Vs Osmolality An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. It is defined as the molarity. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution. Osmotic Pressure Vs Osmolality.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Vs Osmolality Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Concept of osmolality and milliequivalent. Both osmolarity and osmolality are defined in terms of osmoles. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. In order. Osmotic Pressure Vs Osmolality.
From www.slideserve.com
PPT Tonicity, Osmoticity , Osmolarity , & Osmolality PowerPoint Osmotic Pressure Vs Osmolality The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Both osmolarity and osmolality are defined in terms of osmoles. Osmolality (or osmolarity) should. Osmotic Pressure Vs Osmolality.
From ditki.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical Osmotic Pressure Vs Osmolality In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. It is defined as the molarity. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure (\(\pi\)) of. Osmotic Pressure Vs Osmolality.
From www.youtube.com
osmosis, osmotic and hyrostatic pressure, osmolality vs osmolarity Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Concept of osmolality and milliequivalent. It is defined as the molarity. The standard definition of tonicity usually incorporates some mention. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmotic Pressure Vs Osmolality The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Concept of osmolality and milliequivalent. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the two columns. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe. Osmotic Pressure Vs Osmolality.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmotic Pressure Vs Osmolality An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. It is defined as the molarity. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. Both osmolarity and osmolality are defined in terms of osmoles. The. Osmotic Pressure Vs Osmolality.
From droualb.faculty.mjc.edu
Chapter 4 Osmotic Pressure Vs Osmolality Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of. It is defined as the molarity. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable. Osmotic Pressure Vs Osmolality.
From drawittoknowit.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis Draw It to Osmotic Pressure Vs Osmolality It is defined as the molarity. In order to calculate osmotic pressure, it is necessary to understand how solute concentrations are. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments. Osmotic Pressure Vs Osmolality.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Osmotic Pressure Vs Osmolality Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights. Osmotic Pressure Vs Osmolality.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmotic Pressure Vs Osmolality Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The osmotic pressure (\(\pi\)) of the glucose solution is the difference in the pressure between the two sides, in this case the heights of the. Osmotic Pressure Vs Osmolality.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmotic Pressure Vs Osmolality Osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. It is defined as the molarity. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic. Both osmolarity and osmolality are defined in terms of osmoles. The standard definition of. Osmotic Pressure Vs Osmolality.