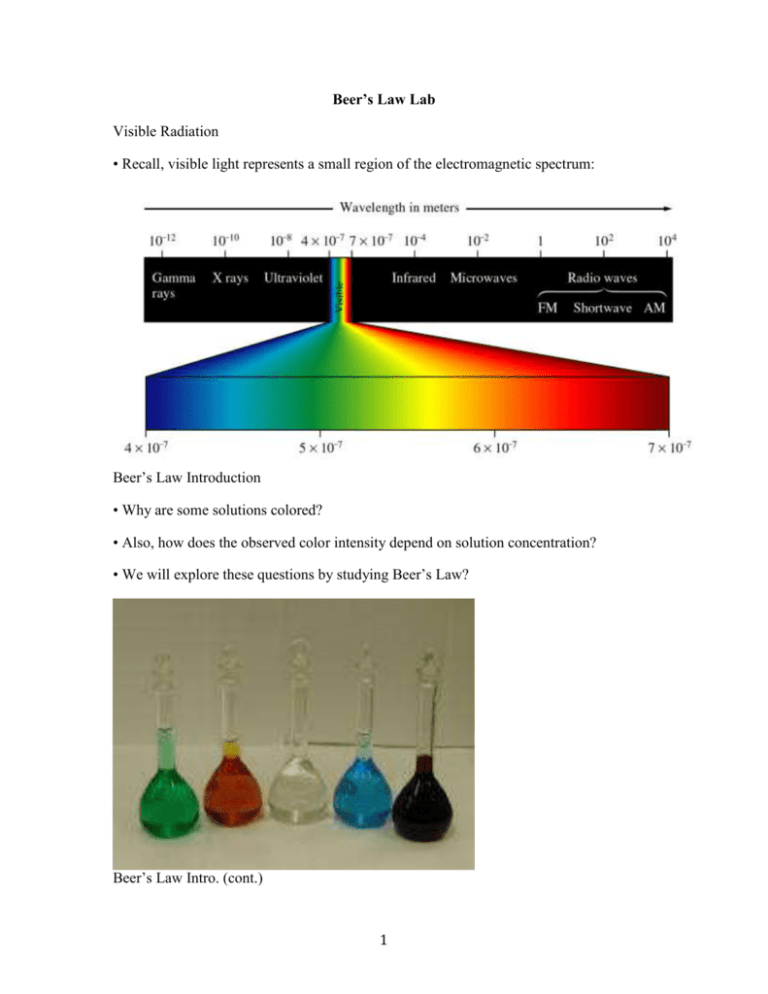
Beer's Law Cuso4 Lab . You will determine the concentration of an unknown cuso. this relationship is known as beer’s law and is given by the equation: since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. concentration for a solution is known as beer’s law (see figure 2). You will determine the concentration of an. the direct relationship between absorbance and concentration for a solution is known as beer’s law. Purpose to determine the wavelength (color) of maximum. Determining the concentration of a solution: make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! determining the concentration of a solution: beer's law and cuso4. A = abc, where a is the absorbance of the. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. The primary objective of this experiment is to determine the.
from studylib.net
You will determine the concentration of an unknown cuso. A = abc, where a is the absorbance of the. Purpose to determine the wavelength (color) of maximum. the direct relationship between absorbance and concentration for a solution is known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. You will determine the concentration of an. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer's law and cuso4. this relationship is known as beer’s law and is given by the equation:
Beer's Law Lab
Beer's Law Cuso4 Lab concentration for a solution is known as beer’s law (see figure 2). A = abc, where a is the absorbance of the. You will determine the concentration of an unknown cuso. The primary objective of this experiment is to determine the. Determining the concentration of a solution: make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. this relationship is known as beer’s law and is given by the equation: beer's law and cuso4. concentration for a solution is known as beer’s law (see figure 2). the direct relationship between absorbance and concentration for a solution is known as beer’s law. Purpose to determine the wavelength (color) of maximum. determining the concentration of a solution: You will determine the concentration of an.
From answerhappy.com
Spectrum of Cu(NH3)4² > (nm) 420 430 440 450 460 470 480 490 Beer's Law Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. A = abc, where a is the absorbance of the. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! You will determine the concentration of an unknown cuso. this relationship is known. Beer's Law Cuso4 Lab.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Cuso4 Lab beer's law and cuso4. the direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the concentration of a solution: based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. The primary objective of this experiment is to determine. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the Beer's Law Cuso4 Lab You will determine the concentration of an. A = abc, where a is the absorbance of the. Purpose to determine the wavelength (color) of maximum. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The primary objective of this experiment is to determine the. concentration for a. Beer's Law Cuso4 Lab.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Cuso4 Lab You will determine the concentration of an unknown cuso. beer's law and cuso4. concentration for a solution is known as beer’s law (see figure 2). Determining the concentration of a solution: Purpose to determine the wavelength (color) of maximum. determining the concentration of a solution: The primary objective of this experiment is to determine the. A =. Beer's Law Cuso4 Lab.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Cuso4 Lab based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. the direct relationship between absorbance and concentration for a solution is known as beer’s law. determining the concentration of a solution: You will determine the concentration of an unknown cuso. Determining the concentration of. Beer's Law Cuso4 Lab.
From studylib.net
Beer`s Law Lab Beer's Law Cuso4 Lab You will determine the concentration of an. The primary objective of this experiment is to determine the. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Cuso4 Lab.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an unknown cuso. Determining the concentration of a solution: A = abc, where a is the absorbance of the. this relationship is known as beer’s law and is given by the equation: The primary objective of this. Beer's Law Cuso4 Lab.
From studylib.net
Beer's Law Lab Beer's Law Cuso4 Lab Purpose to determine the wavelength (color) of maximum. beer's law and cuso4. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = abc, where a is the absorbance of the. The primary objective of this experiment is to determine the. determining the concentration of a. Beer's Law Cuso4 Lab.
From studylib.net
Beer's Law Lab Beer's Law Cuso4 Lab A = abc, where a is the absorbance of the. The primary objective of this experiment is to determine the. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! You will determine the concentration of an. determining the concentration of a solution: You will determine the concentration of. Beer's Law Cuso4 Lab.
From studylib.net
Beer's Law Lab Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer's law and cuso4. A = abc, where a is the absorbance of the. Purpose to determine the wavelength (color) of maximum. . Beer's Law Cuso4 Lab.
From github.com
GitHub phetsims/beerslawlab "Beer's Law Lab" is an educational Beer's Law Cuso4 Lab Determining the concentration of a solution: The primary objective of this experiment is to determine the. A = abc, where a is the absorbance of the. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. You will determine the concentration of an. this relationship. Beer's Law Cuso4 Lab.
From www.bartleby.com
Answered CUSO4 Conc. (M) Absorbance 0.10 0.204… bartleby Beer's Law Cuso4 Lab Determining the concentration of a solution: since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. A = abc, where a is the absorbance of. Beer's Law Cuso4 Lab.
From www.youtube.com
EXPERIMENT LAMBERT BEER'S LAW FOR SOLUTION OF CuSO4 [CHEMISTRT Beer's Law Cuso4 Lab You will determine the concentration of an. determining the concentration of a solution: Determining the concentration of a solution: based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. concentration for a solution is known as beer’s law (see figure 2). Purpose to determine. Beer's Law Cuso4 Lab.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Cuso4 Lab Purpose to determine the wavelength (color) of maximum. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! this relationship is known as beer’s law and is given by the equation: concentration for a solution is known as beer’s law (see figure 2). You will determine the concentration. Beer's Law Cuso4 Lab.
From chemistrysources.com
قانون بيير (بير) Beer's Law التعريف و المعادلة مصادر الكيمياء Beer's Law Cuso4 Lab You will determine the concentration of an. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The primary objective of this experiment is to determine the. Determining the concentration of a solution: concentration for a solution is known as beer’s law (see figure 2). determining the. Beer's Law Cuso4 Lab.
From brainly.com
Problem Based upon the Beer's law plot shown in Figure 2 for the Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. concentration for a solution is known as beer’s law (see figure 2). based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. determining the concentration of a solution:. Beer's Law Cuso4 Lab.
From studylib.net
Determining the Concentration of a Solution Beer’s Law INTRODUCTION Beer's Law Cuso4 Lab this relationship is known as beer’s law and is given by the equation: Purpose to determine the wavelength (color) of maximum. The primary objective of this experiment is to determine the. A = abc, where a is the absorbance of the. the direct relationship between absorbance and concentration for a solution is known as beer’s law. concentration. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved 1. Check Beer's Law by plotting absorbance vs molar Beer's Law Cuso4 Lab You will determine the concentration of an. The primary objective of this experiment is to determine the. this relationship is known as beer’s law and is given by the equation: You will determine the concentration of an unknown cuso. beer's law and cuso4. since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Beer's Law Cuso4 Lab Purpose to determine the wavelength (color) of maximum. You will determine the concentration of an unknown cuso. beer's law and cuso4. The primary objective of this experiment is to determine the. You will determine the concentration of an. determining the concentration of a solution: the direct relationship between absorbance and concentration for a solution is known as. Beer's Law Cuso4 Lab.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Cuso4 Lab You will determine the concentration of an. beer's law and cuso4. concentration for a solution is known as beer’s law (see figure 2). make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! the direct relationship between absorbance and concentration for a solution is known as beer’s. Beer's Law Cuso4 Lab.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer's Law Cuso4 Lab determining the concentration of a solution: this relationship is known as beer’s law and is given by the equation: You will determine the concentration of an. The primary objective of this experiment is to determine the. Determining the concentration of a solution: concentration for a solution is known as beer’s law (see figure 2). You will determine. Beer's Law Cuso4 Lab.
From studylib.net
Determination of Concentration using Colorimetric Analysis & Beer`s Beer's Law Cuso4 Lab based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. determining the concentration of a solution: beer's law and cuso4. Determining the concentration of a solution: Purpose to determine the wavelength (color) of maximum. concentration for a solution is known as beer’s law. Beer's Law Cuso4 Lab.
From www.numerade.com
SOLVED Lab 2 Spectroscopy In Lab, a Beer's Law plot using the Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. A = abc, where a is the absorbance of the. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! determining the concentration of a solution: Determining the concentration of a solution: Purpose. Beer's Law Cuso4 Lab.
From studylib.net
Beer's Law Lab Beer's Law Cuso4 Lab concentration for a solution is known as beer’s law (see figure 2). this relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the. Determining the concentration of a solution: You will determine the concentration of an. beer's law and cuso4. make colorful concentrated and. Beer's Law Cuso4 Lab.
From www.youtube.com
Beer’s Law Lab Analysis YouTube Beer's Law Cuso4 Lab You will determine the concentration of an. Purpose to determine the wavelength (color) of maximum. beer's law and cuso4. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. based on beer's law (a = εcl),4 the slope of the line should be equal to the molar. Beer's Law Cuso4 Lab.
From www.softpedia.com
Download Beer's Law Lab 1.02 Beer's Law Cuso4 Lab The primary objective of this experiment is to determine the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. this relationship is known as beer’s law and is given by the equation: concentration for a solution is known as beer’s law (see figure 2). Determining the. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved Beer's Law 720nm Standard Standard [CuSO4] (M) Beer's Law Cuso4 Lab this relationship is known as beer’s law and is given by the equation: concentration for a solution is known as beer’s law (see figure 2). based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. The primary objective of this experiment is to determine. Beer's Law Cuso4 Lab.
From studylib.net
The BeerLambert Law Beer's Law Cuso4 Lab the direct relationship between absorbance and concentration for a solution is known as beer’s law. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! concentration for a solution is known as beer’s law (see figure 2). A = abc, where a is the absorbance of the. Purpose. Beer's Law Cuso4 Lab.
From www.youtube.com
Verification of Lambert Beers Law By CuSo4 Chemistry Beer's Law Cuso4 Lab concentration for a solution is known as beer’s law (see figure 2). since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The primary objective of this experiment is to determine the. Determining the concentration of a solution: this relationship is known as beer’s law and is. Beer's Law Cuso4 Lab.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Cuso4 Lab A = abc, where a is the absorbance of the. Determining the concentration of a solution: The primary objective of this experiment is to determine the. beer's law and cuso4. You will determine the concentration of an unknown cuso. determining the concentration of a solution: this relationship is known as beer’s law and is given by the. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved Beer's Law 720nm Standard Standard [CuSO4] (M) Beer's Law Cuso4 Lab Purpose to determine the wavelength (color) of maximum. the direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this experiment is to determine the. concentration for a solution is known as beer’s law (see figure 2). determining the concentration of a solution: since the concentration, path length. Beer's Law Cuso4 Lab.
From docslib.org
Beer's Law Lab Determination of the Unknown Concentration of Cuso4 Beer's Law Cuso4 Lab this relationship is known as beer’s law and is given by the equation: based on beer's law (a = εcl),4 the slope of the line should be equal to the molar absorptivity multiplied by the cell. concentration for a solution is known as beer’s law (see figure 2). make colorful concentrated and dilute solutions and explore. Beer's Law Cuso4 Lab.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law Cuso4 Lab A = abc, where a is the absorbance of the. The primary objective of this experiment is to determine the. You will determine the concentration of an unknown cuso. the direct relationship between absorbance and concentration for a solution is known as beer’s law. make colorful concentrated and dilute solutions and explore how much light they absorb and. Beer's Law Cuso4 Lab.
From www.chegg.com
Solved al Chemistry ITLab Beer'sLaw 1. How CuSO4 5H0? Beer's Law Cuso4 Lab A = abc, where a is the absorbance of the. The primary objective of this experiment is to determine the. Purpose to determine the wavelength (color) of maximum. You will determine the concentration of an. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! You will determine the concentration. Beer's Law Cuso4 Lab.
From www.bartleby.com
Answered If you do Beer's law experiment with a… bartleby Beer's Law Cuso4 Lab make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. this relationship is known as beer’s law and is given by the equation: based on beer's law. Beer's Law Cuso4 Lab.