How To Calculate Conductivity Chemistry . Λ m = k / c. To determine of the solution is a strong or weak electrolyte; Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Here, 'k' represents the specific conductivity, and 'c'. To interpret a chemical reaction by observing. Since conductivity is the measure of how easily. To observe electrical conductivity of substances in various aqueous solutions; Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. The mathematical representation of molar conductivity can be expressed using the following formula: Calculating conductivity of a solution given molar conductivities and concentration. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution.
from classnotes.org.in
The mathematical representation of molar conductivity can be expressed using the following formula: Since conductivity is the measure of how easily. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). To interpret a chemical reaction by observing. Λ m = k / c. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Here, 'k' represents the specific conductivity, and 'c'. Calculating conductivity of a solution given molar conductivities and concentration.
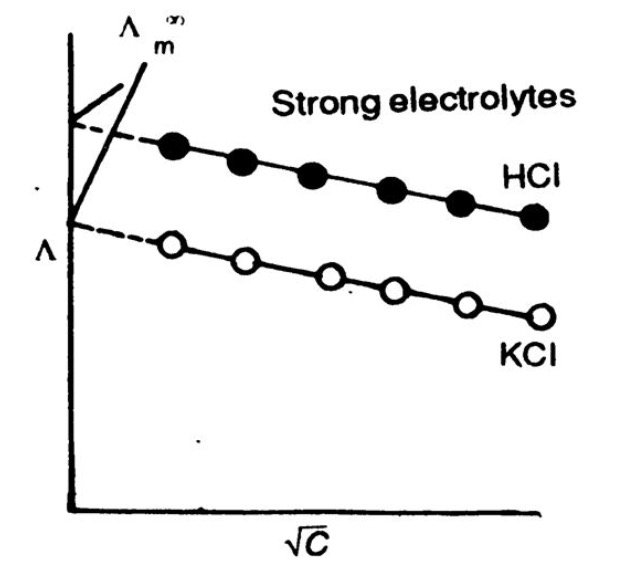
Variation of Conductivity and Molar Conductivity Chemistry, Class 12
How To Calculate Conductivity Chemistry To determine of the solution is a strong or weak electrolyte; Calculating conductivity of a solution given molar conductivities and concentration. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Λ m = k / c. Here, 'k' represents the specific conductivity, and 'c'. The mathematical representation of molar conductivity can be expressed using the following formula: Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Since conductivity is the measure of how easily. To determine of the solution is a strong or weak electrolyte; Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. To interpret a chemical reaction by observing. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). To observe electrical conductivity of substances in various aqueous solutions;
From www.youtube.com
Electrochemistry History and nomenclature, Conductivity, Conductance How To Calculate Conductivity Chemistry Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Calculating conductivity of a solution given molar conductivities and concentration. Here, 'k' represents the specific conductivity, and 'c'. Alpha is equal to. How To Calculate Conductivity Chemistry.
From www.slideserve.com
PPT Ionic Conductivity In A Thermochromic Solid PowerPoint How To Calculate Conductivity Chemistry Calculating conductivity of a solution given molar conductivities and concentration. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Since conductivity is the measure of how easily. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity. How To Calculate Conductivity Chemistry.
From www.youtube.com
Thermal Conductivity Example YouTube How To Calculate Conductivity Chemistry The mathematical representation of molar conductivity can be expressed using the following formula: Calculating conductivity of a solution given molar conductivities and concentration. Λ m = k / c. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Here, 'k' represents the specific conductivity, and 'c'. Molar. How To Calculate Conductivity Chemistry.
From www.electricalcalculators.org
Conductivity Formula and Equation with solved Examples • Electrical How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Calculating conductivity of a solution given molar conductivities and concentration. To interpret a chemical reaction by observing. To observe electrical conductivity of substances in various aqueous solutions; The mathematical representation of molar conductivity can be expressed using the. How To Calculate Conductivity Chemistry.
From byjus.com
from the following molar conductivities at infinite dilution molar How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. Calculating conductivity of a solution given molar conductivities and concentration. To observe electrical conductivity of substances in various aqueous solutions; Λ m = k / c. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). To interpret a chemical reaction. How To Calculate Conductivity Chemistry.
From www.toppr.com
Calculate the equivalent conductivity of a salt solution of 0.75 N How To Calculate Conductivity Chemistry The mathematical representation of molar conductivity can be expressed using the following formula: Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Since conductivity is the measure of how easily. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of. How To Calculate Conductivity Chemistry.
From hxevlantw.blob.core.windows.net
How To Calculate Conductivity Cell Constant at Timothy Quinn blog How To Calculate Conductivity Chemistry To determine of the solution is a strong or weak electrolyte; Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Calculating conductivity of a solution given molar conductivities and concentration. To observe electrical conductivity of substances in various aqueous solutions; Molar conductivity is defined as the conducting power. How To Calculate Conductivity Chemistry.
From byjus.com
50. The conductivity of a solution containing 1 gram of anhydrous BaCl2 How To Calculate Conductivity Chemistry Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. To determine of the solution is a strong or weak electrolyte; Λ m = k / c. To observe electrical conductivity of substances in various aqueous solutions; Since conductivity is the measure of how easily. To interpret a chemical reaction by observing.. How To Calculate Conductivity Chemistry.
From byjus.com
The conductivity of water at 298K is 0.55 × 10^ 7 S cm^ 1. if λ m(H How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. To determine of the solution is a strong or weak electrolyte; Λ m = k / c. Calculating conductivity of a solution given molar conductivities and concentration. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. The mathematical representation. How To Calculate Conductivity Chemistry.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog How To Calculate Conductivity Chemistry The mathematical representation of molar conductivity can be expressed using the following formula: Λ m = k / c. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Here, 'k' represents the specific conductivity, and 'c'. To observe electrical conductivity of substances in various aqueous solutions; Determine the. How To Calculate Conductivity Chemistry.
From classnotes.org.in
Variation of Conductivity and Molar Conductivity Chemistry, Class 12 How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). To determine of the solution is a strong or weak electrolyte; To interpret a chemical reaction. How To Calculate Conductivity Chemistry.
From www.youtube.com
Conductivity of Solutions Demonstration YouTube How To Calculate Conductivity Chemistry To observe electrical conductivity of substances in various aqueous solutions; Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. To determine of the solution is a strong or weak electrolyte; Λ m = k / c. The mathematical representation of molar conductivity can be expressed using the following formula: Since conductivity. How To Calculate Conductivity Chemistry.
From askfilo.com
Calculate the conductance, conductivity and molar conductivity of a 0.01M.. How To Calculate Conductivity Chemistry To interpret a chemical reaction by observing. Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Here, 'k' represents the specific conductivity, and 'c'. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Determine the limiting molar. How To Calculate Conductivity Chemistry.
From byjus.com
calculate molar conductivityof oxalic acid, given that,Na2C2O4 400 Ω How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Here, 'k' represents the specific conductivity, and 'c'. Λ m = k / c. Since conductivity is the. How To Calculate Conductivity Chemistry.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download How To Calculate Conductivity Chemistry To observe electrical conductivity of substances in various aqueous solutions; To determine of the solution is a strong or weak electrolyte; Calculating conductivity of a solution given molar conductivities and concentration. Here, 'k' represents the specific conductivity, and 'c'. Since conductivity is the measure of how easily. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate,. How To Calculate Conductivity Chemistry.
From www.youtube.com
Specific Conductance, Conductivity Conductance units of conductivity How To Calculate Conductivity Chemistry Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Λ m = k / c. To interpret a chemical reaction by observing. To observe electrical conductivity of substances in various aqueous solutions;. How To Calculate Conductivity Chemistry.
From www.youtube.com
How To calculate Conductivity using EIS YouTube How To Calculate Conductivity Chemistry Calculating conductivity of a solution given molar conductivities and concentration. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). The mathematical representation of molar conductivity can be expressed using the following formula: Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Λ. How To Calculate Conductivity Chemistry.
From byjus.com
The conductivity of pure water is 5.5×10^ 8 ohm^ 1 cm^ 1. The How To Calculate Conductivity Chemistry The mathematical representation of molar conductivity can be expressed using the following formula: Here, 'k' represents the specific conductivity, and 'c'. To observe electrical conductivity of substances in various aqueous solutions; Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). To determine of the solution is a strong or weak electrolyte; Molar conductivity is. How To Calculate Conductivity Chemistry.
From www.youtube.com
Calculating conductivity and molar conductivity Resistance of a How To Calculate Conductivity Chemistry Λ m = k / c. To determine of the solution is a strong or weak electrolyte; To observe electrical conductivity of substances in various aqueous solutions; Calculating conductivity of a solution given molar conductivities and concentration. The mathematical representation of molar conductivity can be expressed using the following formula: Since conductivity is the measure of how easily. Conductivity is. How To Calculate Conductivity Chemistry.
From byjus.com
how does the conductivity and molar conductivity varies with concentration How To Calculate Conductivity Chemistry Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Here, 'k' represents the specific conductivity, and 'c'. Alpha is equal to the molar conductivity of the species. How To Calculate Conductivity Chemistry.
From sciencing.com
How to Calculate Conductivity Due to Concentration Sciencing How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. Λ m = k / c. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Calculating conductivity of a solution given molar conductivities and concentration. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride. How To Calculate Conductivity Chemistry.
From www.youtube.com
Chemistry 12 Chapter 3 Electrochemistry Conductance, Conductivity How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. To determine of the solution is a strong or weak electrolyte; To interpret a chemical reaction by observing. The mathematical. How To Calculate Conductivity Chemistry.
From www.chegg.com
Solved Using the follow formula for conductivity, prove/show How To Calculate Conductivity Chemistry Calculating conductivity of a solution given molar conductivities and concentration. Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Λ m = k / c. Alpha is equal to the molar conductivity of the. How To Calculate Conductivity Chemistry.
From www.youtube.com
MOLAR AND EQUIVALENT CONDUCTIVITY YouTube How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. Calculating conductivity of a solution given molar conductivities and concentration. Here, 'k' represents the specific conductivity, and 'c'. To observe electrical conductivity of substances in various aqueous solutions; Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. The mathematical representation of. How To Calculate Conductivity Chemistry.
From www.youtube.com
Molar Conductivity and Equivalent Conductivity Class 12 Chemistry How To Calculate Conductivity Chemistry Calculating conductivity of a solution given molar conductivities and concentration. To interpret a chemical reaction by observing. To observe electrical conductivity of substances in various aqueous solutions; Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. The mathematical representation of molar conductivity can be expressed using the. How To Calculate Conductivity Chemistry.
From www.toppr.com
Calculate molar conductance for NH4OH , given that molar conductances How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Since conductivity is the measure of how easily. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Molar conductivity is the property used in determining the efficiency of a given electrolyte in. How To Calculate Conductivity Chemistry.
From www.youtube.com
Problem 1 on molar conductivity (Electrochemistry part 54 for CBSE How To Calculate Conductivity Chemistry To determine of the solution is a strong or weak electrolyte; Λ m = k / c. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Since conductivity is the measure of how easily. To observe electrical conductivity of substances in various aqueous solutions; Here, 'k' represents the specific conductivity, and 'c'. Molar conductivity. How To Calculate Conductivity Chemistry.
From www.youtube.com
Calculate the conductance and conductivity of a wire of radius `0.01 How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute molar conductivity (constant). Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric. How To Calculate Conductivity Chemistry.
From www.youtube.com
Molar conductivity (Electrochemistry part 53 for CBSE class 12 JEE IIT How To Calculate Conductivity Chemistry Molar conductivity is defined as the conducting power of ions that are formed by dissolving a mole of electrolyte in a solution. Calculating conductivity of a solution given molar conductivities and concentration. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Λ m = k / c. To interpret a chemical reaction by observing.. How To Calculate Conductivity Chemistry.
From www.youtube.com
How to Calculate the activation energy from DC and AC conductivity How To Calculate Conductivity Chemistry Since conductivity is the measure of how easily. To observe electrical conductivity of substances in various aqueous solutions; Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. To determine of the solution is a strong or weak electrolyte; Here, 'k' represents the specific conductivity, and 'c'. The mathematical representation. How To Calculate Conductivity Chemistry.
From www.youtube.com
Variation of Conductivity and Molar Conductivity with Concentration How To Calculate Conductivity Chemistry Λ m = k / c. Calculating conductivity of a solution given molar conductivities and concentration. Here, 'k' represents the specific conductivity, and 'c'. Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Alpha is equal to the molar conductivity of the species at a particular concentration divided by the absolute. How To Calculate Conductivity Chemistry.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific How To Calculate Conductivity Chemistry Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). To determine of the solution is a strong or weak electrolyte; Here, 'k' represents the specific conductivity, and 'c'. Alpha is equal to the molar. How To Calculate Conductivity Chemistry.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University How To Calculate Conductivity Chemistry Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Λ m = k / c. Molar conductivity is the property used in determining the efficiency of a given electrolyte in conducting electricity in a solution. Molar conductivity is defined as the conducting power of ions that are formed by dissolving a. How To Calculate Conductivity Chemistry.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog How To Calculate Conductivity Chemistry Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Since conductivity is the measure of how easily. The mathematical representation of molar conductivity can be expressed using the following formula: Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. Here, 'k' represents the specific conductivity,. How To Calculate Conductivity Chemistry.
From www.youtube.com
How to Calculate the Conductivity, Hole Mobility and IV Curves YouTube How To Calculate Conductivity Chemistry Determine the limiting molar conductivity \lambda_\infty and the factor b for sodium acetate, sodium chloride and hydrochloric acid. To interpret a chemical reaction by observing. Here, 'k' represents the specific conductivity, and 'c'. Λ m = k / c. Conductivity is represented by \ ( \sigma \) and is measured in siemens (\ (1/ωm\)). Since conductivity is the measure of. How To Calculate Conductivity Chemistry.