In A Solid To Liquid Mixture An Increase In Temperature Means . In a solid to liquid mixture, an increase in temperature means. A solubility curve is a graph of the solubility vs. This diagram can be used to explain several kinds of phenomenon. Increasing the temperature always decreases. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. Gas solubility decreases as the temperature increases. Suppose a liquid solution of with a mole fraction. Gas solubility decreases as the temperature. The solubility of a solid in water increases with an increase in temperature. The solubility of a solid in water increases with an increase in temperature. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. However, this is not a hard and fast rule. 【solved】click here to get an answer to your question : Below this temperature, any mixture of ice and acetic acid is solid. Usually, increasing the temperature increases the solubility of solids and liquids.
from www.teachoo.com
Suppose a liquid solution of with a mole fraction. Usually, increasing the temperature increases the solubility of solids and liquids. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. Below this temperature, any mixture of ice and acetic acid is solid. However, this is not a hard and fast rule. Gas solubility decreases as the temperature increases. A solubility curve is a graph of the solubility vs. In a solid to liquid mixture, an increase in temperature means. Increasing the temperature always decreases. The solubility of a solid in water increases with an increase in temperature.
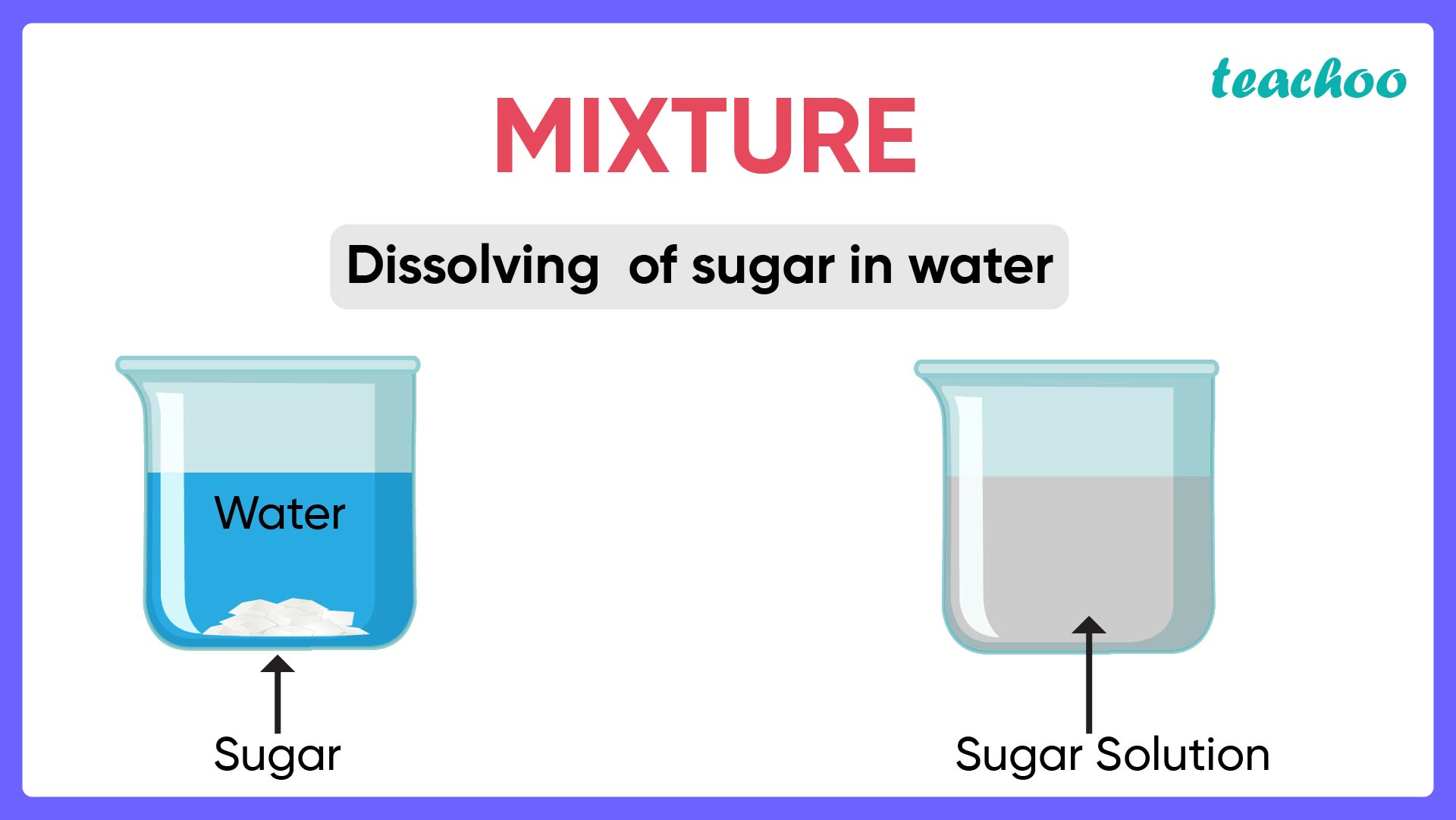
What is a Mixture? Types of Mixtures Chemistry Teachoo
In A Solid To Liquid Mixture An Increase In Temperature Means Increasing the temperature always decreases. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. This diagram can be used to explain several kinds of phenomenon. Suppose a liquid solution of with a mole fraction. Usually, increasing the temperature increases the solubility of solids and liquids. The solubility of a solid in water increases with an increase in temperature. In a solid to liquid mixture, an increase in temperature means. Gas solubility decreases as the temperature increases. A solubility curve is a graph of the solubility vs. Increasing the temperature always decreases. Below this temperature, any mixture of ice and acetic acid is solid. The solubility of a solid in water increases with an increase in temperature. 【solved】click here to get an answer to your question : However, this is not a hard and fast rule. Gas solubility decreases as the temperature. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk In A Solid To Liquid Mixture An Increase In Temperature Means Increasing the temperature always decreases. A solubility curve is a graph of the solubility vs. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. 【solved】click here to get an answer to your question : Usually, increasing the temperature increases the solubility of solids and liquids. This diagram can. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.thoughtco.com
Mixture Definition and Examples in Science In A Solid To Liquid Mixture An Increase In Temperature Means Suppose a liquid solution of with a mole fraction. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Usually, increasing the temperature increases the solubility of solids and liquids. Gas solubility decreases as the temperature increases. However, this is not a hard and fast rule. The solubility of. In A Solid To Liquid Mixture An Increase In Temperature Means.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas In A Solid To Liquid Mixture An Increase In Temperature Means Increasing the temperature always decreases. 【solved】click here to get an answer to your question : In a solid to liquid mixture, an increase in temperature means. However, this is not a hard and fast rule. Below this temperature, any mixture of ice and acetic acid is solid. Usually, increasing the temperature increases the solubility of solids and liquids. The solubility. In A Solid To Liquid Mixture An Increase In Temperature Means.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps In A Solid To Liquid Mixture An Increase In Temperature Means Suppose a liquid solution of with a mole fraction. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. A solubility curve is a graph of the solubility vs. In a solid to liquid mixture, an increase in temperature means. This diagram can be used. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.slideserve.com
PPT Review of Basic Principle of Thermodynamics 1 PowerPoint In A Solid To Liquid Mixture An Increase In Temperature Means Below this temperature, any mixture of ice and acetic acid is solid. Increasing the temperature always decreases. A solubility curve is a graph of the solubility vs. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. The solubility of a solid in water increases. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.animalia-life.club
Examples Of Solids Liquids And Gases In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature. Gas solubility decreases as the temperature increases. A solubility curve is a graph of the solubility vs. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Suppose a liquid solution of with a mole fraction. The solubility of a solid in water. In A Solid To Liquid Mixture An Increase In Temperature Means.
From saylordotorg.github.io
Properties of Liquids In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature increases. In a solid to liquid mixture, an increase in temperature means. The solubility of a solid in water increases with an increase in temperature. However, this is not a hard and fast rule. 【solved】click here to get an answer to your question : The solubility of a solid in water increases with an. In A Solid To Liquid Mixture An Increase In Temperature Means.
From courses.lumenlearning.com
Phase Changes Physics In A Solid To Liquid Mixture An Increase In Temperature Means However, this is not a hard and fast rule. In a solid to liquid mixture, an increase in temperature means. The solubility of a solid in water increases with an increase in temperature. This diagram can be used to explain several kinds of phenomenon. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of. In A Solid To Liquid Mixture An Increase In Temperature Means.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change In A Solid To Liquid Mixture An Increase In Temperature Means 【solved】click here to get an answer to your question : The solubility of a solid in water increases with an increase in temperature. Gas solubility decreases as the temperature. This diagram can be used to explain several kinds of phenomenon. Usually, increasing the temperature increases the solubility of solids and liquids. Suppose a liquid solution of with a mole fraction.. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The In A Solid To Liquid Mixture An Increase In Temperature Means A solubility curve is a graph of the solubility vs. The solubility of a solid in water increases with an increase in temperature. The solubility of a solid in water increases with an increase in temperature. In a solid to liquid mixture, an increase in temperature means. Below this temperature, any mixture of ice and acetic acid is solid. For. In A Solid To Liquid Mixture An Increase In Temperature Means.
From ecurrencythailand.com
What Is The Effect Of Temperature On The Solubility Of A Solid In A In A Solid To Liquid Mixture An Increase In Temperature Means In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. Gas solubility decreases as the temperature. Gas solubility decreases as the temperature increases. However, this is not a hard and fast rule. Suppose a liquid solution of with a mole fraction. The solubility of a. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature. This diagram can be used to explain several kinds of phenomenon. Increasing the temperature always decreases. A solubility curve is a graph of the solubility vs. Below this temperature, any mixture of ice and acetic acid is solid. Suppose a liquid solution of with a mole fraction. The solubility of a solid in water. In A Solid To Liquid Mixture An Increase In Temperature Means.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. Gas solubility decreases as the temperature increases. A solubility curve is a graph of the solubility vs. For many solid solutes in liquid solvents (as we see from everyday. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to In A Solid To Liquid Mixture An Increase In Temperature Means For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Increasing the temperature always decreases. In a solid to liquid mixture, an increase in temperature means. 【solved】click here to get an answer to your question : This diagram can be used to explain several kinds of phenomenon. Below this. In A Solid To Liquid Mixture An Increase In Temperature Means.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases In A Solid To Liquid Mixture An Increase In Temperature Means This diagram can be used to explain several kinds of phenomenon. Gas solubility decreases as the temperature. The solubility of a solid in water increases with an increase in temperature. However, this is not a hard and fast rule. Gas solubility decreases as the temperature increases. Below this temperature, any mixture of ice and acetic acid is solid. Usually, increasing. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science In A Solid To Liquid Mixture An Increase In Temperature Means This diagram can be used to explain several kinds of phenomenon. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. In a solid to liquid mixture, an increase in temperature means. For many solid solutes in liquid solvents (as we see from everyday life). In A Solid To Liquid Mixture An Increase In Temperature Means.
From mungfali.com
Solids Liquids Gases Chart In A Solid To Liquid Mixture An Increase In Temperature Means The solubility of a solid in water increases with an increase in temperature. Gas solubility decreases as the temperature. This diagram can be used to explain several kinds of phenomenon. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. Usually, increasing the temperature increases. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.snexplores.org
Explainer What are the different states of matter? In A Solid To Liquid Mixture An Increase In Temperature Means The solubility of a solid in water increases with an increase in temperature. However, this is not a hard and fast rule. Gas solubility decreases as the temperature increases. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Usually, increasing the temperature increases the solubility of solids and. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.youtube.com
Specific heat capacity of solid by the method of mixture YouTube In A Solid To Liquid Mixture An Increase In Temperature Means Usually, increasing the temperature increases the solubility of solids and liquids. Increasing the temperature always decreases. The solubility of a solid in water increases with an increase in temperature. 【solved】click here to get an answer to your question : Suppose a liquid solution of with a mole fraction. For many solid solutes in liquid solvents (as we see from everyday. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.youtube.com
Changes in Materials when Mixed with other Materials (Part 3 Solid to In A Solid To Liquid Mixture An Increase In Temperature Means This diagram can be used to explain several kinds of phenomenon. The solubility of a solid in water increases with an increase in temperature. In a solid to liquid mixture, an increase in temperature means. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature increases. Usually, increasing the temperature increases the solubility of. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to In A Solid To Liquid Mixture An Increase In Temperature Means In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. 【solved】click here to get an answer to your question : In a solid to liquid mixture, an increase in temperature means. The solubility of a solid in water increases with an increase in temperature. For. In A Solid To Liquid Mixture An Increase In Temperature Means.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature. Gas solubility decreases as the temperature increases. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. However, this is not a hard and fast rule. Below this temperature, any mixture of ice and acetic acid is solid. A. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube In A Solid To Liquid Mixture An Increase In Temperature Means This diagram can be used to explain several kinds of phenomenon. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. 【solved】click here to get an answer to your question : Gas solubility decreases as the temperature increases. Suppose a liquid solution of with a mole fraction. In a. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.youtube.com
Finding the Equilibrium Temperature of a Mixture of Different In A Solid To Liquid Mixture An Increase In Temperature Means A solubility curve is a graph of the solubility vs. Increasing the temperature always decreases. This diagram can be used to explain several kinds of phenomenon. 【solved】click here to get an answer to your question : Below this temperature, any mixture of ice and acetic acid is solid. For many solid solutes in liquid solvents (as we see from everyday. In A Solid To Liquid Mixture An Increase In Temperature Means.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk In A Solid To Liquid Mixture An Increase In Temperature Means 【solved】click here to get an answer to your question : For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. However, this is not a hard and fast rule. Gas solubility decreases as the temperature. In a solid to liquid mixture, an increase in temperature means. The solubility of. In A Solid To Liquid Mixture An Increase In Temperature Means.
From chem.libretexts.org
Thermodynamics of Mixing Chemistry LibreTexts In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature. Increasing the temperature always decreases. This diagram can be used to explain several kinds of phenomenon. The solubility of a solid in water increases with an increase in temperature. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Suppose a liquid solution. In A Solid To Liquid Mixture An Increase In Temperature Means.
From socratic.org
What are examples of gases, liquids, and solids? Socratic In A Solid To Liquid Mixture An Increase In Temperature Means The solubility of a solid in water increases with an increase in temperature. Increasing the temperature always decreases. Below this temperature, any mixture of ice and acetic acid is solid. Gas solubility decreases as the temperature increases. In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube In A Solid To Liquid Mixture An Increase In Temperature Means Increasing the temperature always decreases. Gas solubility decreases as the temperature. The solubility of a solid in water increases with an increase in temperature. 【solved】click here to get an answer to your question : This diagram can be used to explain several kinds of phenomenon. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the. In A Solid To Liquid Mixture An Increase In Temperature Means.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 In A Solid To Liquid Mixture An Increase In Temperature Means In a mixture of solid and liquid at equilibrium, the reciprocal processes of melting and freezing occur at equal rates, and the quantities of solid. However, this is not a hard and fast rule. Suppose a liquid solution of with a mole fraction. Below this temperature, any mixture of ice and acetic acid is solid. The solubility of a solid. In A Solid To Liquid Mixture An Increase In Temperature Means.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES In A Solid To Liquid Mixture An Increase In Temperature Means Usually, increasing the temperature increases the solubility of solids and liquids. In a solid to liquid mixture, an increase in temperature means. This diagram can be used to explain several kinds of phenomenon. A solubility curve is a graph of the solubility vs. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.solnpharma.com
Difference Between Solid and Liquid Mixing In A Solid To Liquid Mixture An Increase In Temperature Means For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. This diagram can be used to explain several kinds of phenomenon. Gas solubility decreases as the temperature increases. Increasing the temperature always decreases. The solubility of a solid in water increases with an increase in temperature. 【solved】click here to. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.britannica.com
phase Definition & Facts Britannica In A Solid To Liquid Mixture An Increase In Temperature Means Gas solubility decreases as the temperature increases. This diagram can be used to explain several kinds of phenomenon. Suppose a liquid solution of with a mole fraction. For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. In a solid to liquid mixture, an increase in temperature means. A. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.researchgate.net
Solidliquid temperature composition phase diagram for a binary system In A Solid To Liquid Mixture An Increase In Temperature Means However, this is not a hard and fast rule. Usually, increasing the temperature increases the solubility of solids and liquids. This diagram can be used to explain several kinds of phenomenon. Below this temperature, any mixture of ice and acetic acid is solid. In a solid to liquid mixture, an increase in temperature means. The solubility of a solid in. In A Solid To Liquid Mixture An Increase In Temperature Means.
From www.teachoo.com
What is a Mixture? Types of Mixtures Chemistry Teachoo In A Solid To Liquid Mixture An Increase In Temperature Means For many solid solutes in liquid solvents (as we see from everyday life) the solubility of the solute increases with temperature. Suppose a liquid solution of with a mole fraction. The solubility of a solid in water increases with an increase in temperature. Gas solubility decreases as the temperature. A solubility curve is a graph of the solubility vs. In. In A Solid To Liquid Mixture An Increase In Temperature Means.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary In A Solid To Liquid Mixture An Increase In Temperature Means Below this temperature, any mixture of ice and acetic acid is solid. The solubility of a solid in water increases with an increase in temperature. Suppose a liquid solution of with a mole fraction. Increasing the temperature always decreases. The solubility of a solid in water increases with an increase in temperature. 【solved】click here to get an answer to your. In A Solid To Liquid Mixture An Increase In Temperature Means.