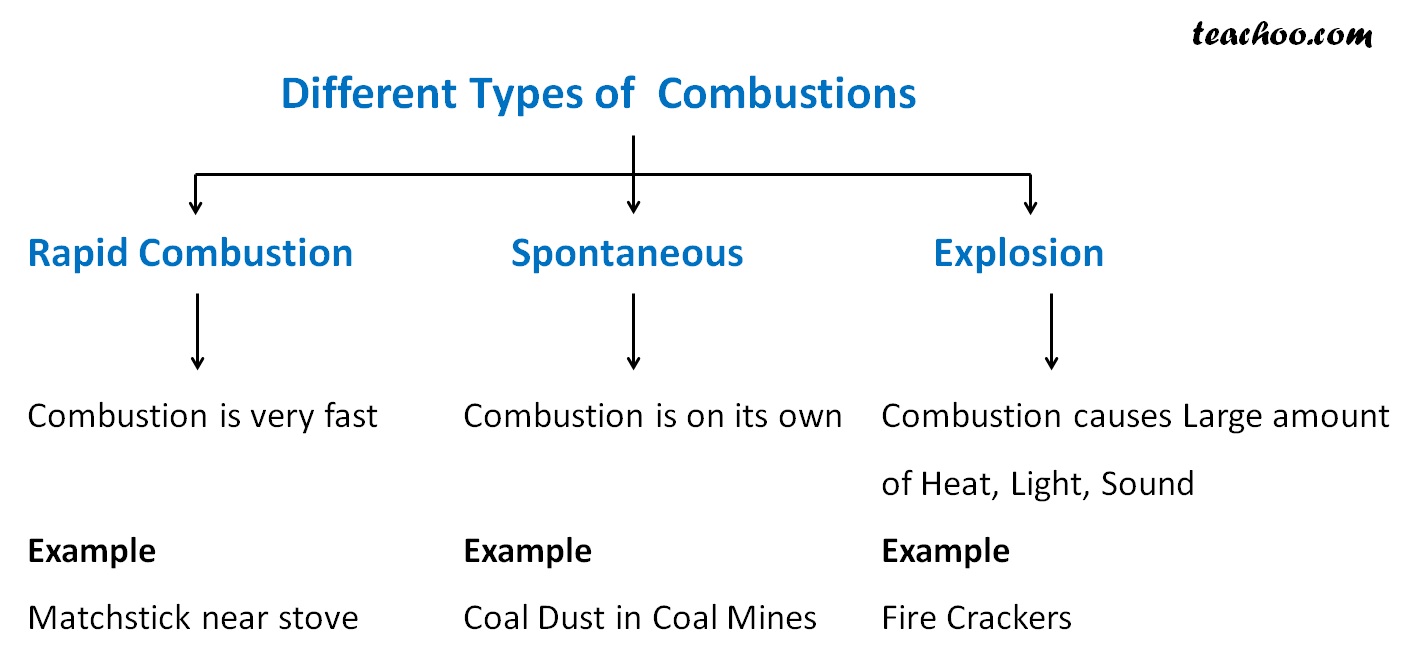
What Are The 3 Types Of Combustion . Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Two types of hydrocarbon combustion have been defined:
from www.teachoo.com
Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Two types of hydrocarbon combustion have been defined: Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,.
Different Types of Combustion with Examples Teachoo Concepts
What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Two types of hydrocarbon combustion have been defined: Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,.
From www.slideserve.com
PPT CHAPTER 6 COMBUSTION AND FLAME PowerPoint Presentation, free What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Flame combustion is most prominent with fuels. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. What Are The 3 Types Of Combustion.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Throughout this article, we explored various types. What Are The 3 Types Of Combustion.
From www.teachoo.com
What is Combustion? Explained with reactions Teachoo What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Two types of hydrocarbon combustion have been. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Combustion and flame PowerPoint Presentation, free download ID What Are The 3 Types Of Combustion Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed. What Are The 3 Types Of Combustion.
From www.youtube.com
Balancing Combustion Reactions YouTube What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. (1) slow combustion at. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Combustion Theory (Definition, influencing factor etc What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. What Are The 3 Types Of Combustion.
From www.scribd.com
3 Types of Combustion PDF PDF What Are The 3 Types Of Combustion Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed. What Are The 3 Types Of Combustion.
From 88guru.com
Types of Chemical Reactions Combustion, Displacement & What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g.,. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Fuels PowerPoint Presentation, free download ID6911564 What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co. What Are The 3 Types Of Combustion.
From mavink.com
Different Types Of Combustion What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. What Are The 3 Types Of Combustion.
From sciencenotes.org
Combustion Reaction Definition and Examples What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at temperatures below 500. What Are The 3 Types Of Combustion.
From engineeringlearner.com
Types of Combustion Chamber Functions, Advantages & Disadvantages What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Two types of hydrocarbon combustion have been defined: (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Flame combustion is most prominent with fuels that have been premixed with an oxidant,. What Are The 3 Types Of Combustion.
From mavink.com
Different Types Of Combustion What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing. What Are The 3 Types Of Combustion.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. What Are The 3 Types Of Combustion.
From cfdyna.com
Combustion Simulation in OpenFOAM What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. What Are The 3 Types Of Combustion.
From www.youtube.com
TYPES OF COMBUSTION Combustion and Flame Rapid Spontaneous What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood,. What Are The 3 Types Of Combustion.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at. What Are The 3 Types Of Combustion.
From www.youtube.com
Combustion and Flame YouTube What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Two types of hydrocarbon combustion have been defined: Throughout this article, we explored various types of combustion, such as complete combustion, incomplete. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Lesson PowerPoint Presentation, free download ID5359562 What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Throughout this article, we explored various types of combustion, such as complete. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT The Combustion Process PowerPoint Presentation, free download What Are The 3 Types Of Combustion Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Two types of hydrocarbon combustion have been defined: (1) slow combustion at. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT CHAPTER 6 COMBUSTION AND FLAME PowerPoint Presentation, free What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion,. What Are The 3 Types Of Combustion.
From www.teachoo.com
Different Types of Combustion with Examples Teachoo Concepts What Are The 3 Types Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Throughout this article, we explored various types of combustion, such as complete. What Are The 3 Types Of Combustion.
From www.studocu.com
What are Different Types of Combustion Chamber What are Different What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion is a. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free What Are The 3 Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Two types of hydrocarbon combustion have been defined: Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the. What Are The 3 Types Of Combustion.
From www.grc.nasa.gov
Combustion What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion is a reaction between a hydrocarbon fuel (e.g.,. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT chemistry of fire PowerPoint Presentation, free download ID1129778 What Are The 3 Types Of Combustion Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and. What Are The 3 Types Of Combustion.
From www.pinterest.co.kr
Combustion Easy Science Learn physics, Physics concepts, Easy science What Are The 3 Types Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Reaction Prediction PowerPoint Presentation, free download ID What Are The 3 Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Throughout this article, we explored various types of combustion, such as complete combustion, incomplete combustion, spontaneous combustion, rapid combustion,. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. What Are The 3 Types Of Combustion.
From www.slideserve.com
PPT Types of Chemical Reactions (rxns.) PowerPoint Presentation, free What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of. What Are The 3 Types Of Combustion.
From www.youtube.com
Science 8 Chapter 6 Control of Fire, Types of Combustion, History of What Are The 3 Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or. What Are The 3 Types Of Combustion.
From slideplayer.com
What we will do What we will learn 10C 27 WhiringaāNuku ppt download What Are The 3 Types Of Combustion Two types of hydrocarbon combustion have been defined: Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Throughout this article, we. What Are The 3 Types Of Combustion.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES What Are The 3 Types Of Combustion (1) slow combustion at temperatures below 500 °c, including cool flames observed at certain. Flame combustion is most prominent with fuels that have been premixed with an oxidant, either oxygen or a compound that provides oxygen, for the. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form. What Are The 3 Types Of Combustion.