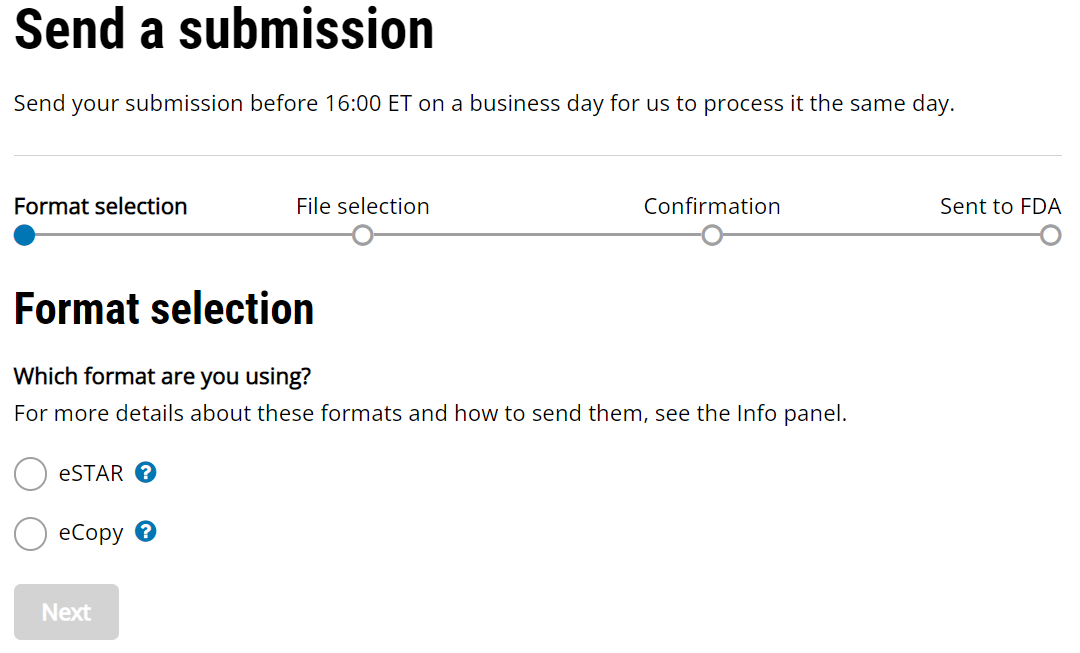
Fda Medical Device Electronic Instructions For Use . reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. 21 cfr part 801 and 809. format instructions for use (eifu) for all professional use medical devices. The fda has a series of requirements for the instructions for use; In 21 cfr part 801 for medical devices and in. manufacturers who want to provide instructions for use in electronic form should study the requirements of. Industry has been asked at. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic.
from medicaldeviceacademy.com
manufacturers who want to provide instructions for use in electronic form should study the requirements of. format instructions for use (eifu) for all professional use medical devices. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. Industry has been asked at. 21 cfr part 801 and 809. The fda has a series of requirements for the instructions for use; reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in.
510k Electronic Submission Guidance for FDA 510k Submissions
Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. Industry has been asked at. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in. The fda has a series of requirements for the instructions for use; manufacturers who want to provide instructions for use in electronic form should study the requirements of. 21 cfr part 801 and 809. format instructions for use (eifu) for all professional use medical devices. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. The fda has a series of requirements for the instructions for use; Industry has been asked at. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. In 21 cfr part 801 for medical devices and in. . Fda Medical Device Electronic Instructions For Use.
From medicaldeviceacademy.com
510k Electronic Submission Guidance for FDA 510k Submissions Fda Medical Device Electronic Instructions For Use In 21 cfr part 801 for medical devices and in. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. Industry has been asked at. 21 cfr part 801 and 809. format instructions for use (eifu) for all professional use medical devices. manufacturers who want to provide instructions for use in electronic. Fda Medical Device Electronic Instructions For Use.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Fda Medical Device Electronic Instructions For Use format instructions for use (eifu) for all professional use medical devices. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. Industry has been asked at. manufacturers who want to provide instructions for use in electronic. Fda Medical Device Electronic Instructions For Use.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. manufacturers who want to provide instructions for use in electronic form should study the requirements of. In 21 cfr part 801 for medical devices and in. . Fda Medical Device Electronic Instructions For Use.
From www.regdesk.co
FDA Draft Guidance on Electronic Submission Template for Medical Device Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. The fda has a series of requirements for the instructions for use; In 21 cfr part 801 for medical devices and in. format instructions for use (eifu) for all professional use medical devices. Industry has been asked at. fda has developed. Fda Medical Device Electronic Instructions For Use.
From digitalhealth.folio3.com
FDA Software Guide FDA software as a medical device Fda Medical Device Electronic Instructions For Use Industry has been asked at. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. 21 cfr part 801 and 809.. Fda Medical Device Electronic Instructions For Use.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Criteria 13 for Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in. . Fda Medical Device Electronic Instructions For Use.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Fda Medical Device Electronic Instructions For Use In 21 cfr part 801 for medical devices and in. 21 cfr part 801 and 809. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. format instructions for use (eifu) for all professional use medical devices. Industry has been asked at. manufacturers who want to provide instructions for use in electronic. Fda Medical Device Electronic Instructions For Use.
From www.regdesk.co
FDA Guidance on Electronic Medical Device Reporting (eMDR) RegDesk Fda Medical Device Electronic Instructions For Use The fda has a series of requirements for the instructions for use; format instructions for use (eifu) for all professional use medical devices. 21 cfr part 801 and 809. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in. manufacturers who want. Fda Medical Device Electronic Instructions For Use.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers Fda Medical Device Electronic Instructions For Use In 21 cfr part 801 for medical devices and in. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. 21 cfr part 801 and 809. Industry has been asked at. format instructions for use (eifu) for. Fda Medical Device Electronic Instructions For Use.
From exyuxzjps.blob.core.windows.net
Medical Device Regulations Fda at Lois Ogrady blog Fda Medical Device Electronic Instructions For Use Industry has been asked at. format instructions for use (eifu) for all professional use medical devices. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers. Fda Medical Device Electronic Instructions For Use.
From www.exeedqm.com
FDA Guidance Shows a Regulatory Path Forward for Interoperable Devices Fda Medical Device Electronic Instructions For Use 21 cfr part 801 and 809. Industry has been asked at. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. manufacturers who want to provide instructions for use in electronic form should study the requirements of. In 21 cfr part 801 for medical devices and in. The fda has a series of. Fda Medical Device Electronic Instructions For Use.
From blog.sierralabs.com
A Simple Guide to 510(k) Applications for Medical Devices Fda Medical Device Electronic Instructions For Use The fda has a series of requirements for the instructions for use; 21 cfr part 801 and 809. Industry has been asked at. manufacturers who want to provide instructions for use in electronic form should study the requirements of. In 21 cfr part 801 for medical devices and in. format instructions for use (eifu) for all professional use. Fda Medical Device Electronic Instructions For Use.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Medical Device Electronic Instructions For Use Industry has been asked at. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. The fda has a series of requirements for the instructions for use; manufacturers who want to provide instructions for use in electronic form should study the requirements of. In 21 cfr part 801 for medical devices and in.. Fda Medical Device Electronic Instructions For Use.
From www.presentationeze.com
Medical Device Instructions for Use Information & TrainingPresentationEZE Fda Medical Device Electronic Instructions For Use In 21 cfr part 801 for medical devices and in. format instructions for use (eifu) for all professional use medical devices. The fda has a series of requirements for the instructions for use; 21 cfr part 801 and 809. manufacturers who want to provide instructions for use in electronic form should study the requirements of. reading the. Fda Medical Device Electronic Instructions For Use.
From www.hcinnovationgroup.com
UDI Not just for manufacturers anymore Healthcare Innovation Fda Medical Device Electronic Instructions For Use fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. Industry has been asked at. format instructions for use (eifu) for all professional use medical devices. 21 cfr part 801 and 809. In 21 cfr part 801 for medical devices and in. The fda has a series of requirements for the instructions for. Fda Medical Device Electronic Instructions For Use.
From www.waysps.com
New Draft FDA Guidance Electronic Format for Medical Device Submissions Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. 21 cfr part 801 and 809. format instructions for use (eifu) for all professional use medical devices. Industry has been asked at. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part. Fda Medical Device Electronic Instructions For Use.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Criterion 4 5 for Fda Medical Device Electronic Instructions For Use The fda has a series of requirements for the instructions for use; In 21 cfr part 801 for medical devices and in. Industry has been asked at. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of.. Fda Medical Device Electronic Instructions For Use.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Medical Device Electronic Instructions For Use 21 cfr part 801 and 809. Industry has been asked at. format instructions for use (eifu) for all professional use medical devices. In 21 cfr part 801 for medical devices and in. The fda has a series of requirements for the instructions for use; reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226. Fda Medical Device Electronic Instructions For Use.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Fda Medical Device Electronic Instructions For Use 21 cfr part 801 and 809. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. format instructions for use (eifu) for all professional use medical devices. manufacturers who want to provide instructions for use in electronic form should study the requirements of. The fda has a series of requirements for the. Fda Medical Device Electronic Instructions For Use.
From www.access.fda.gov
Device Registration and Listing Module (DRLM) StepbyStep Instructions Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. The fda has a series of requirements for the instructions for use; In 21 cfr part 801 for medical devices and in. Industry has been asked at.. Fda Medical Device Electronic Instructions For Use.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Fda Medical Device Electronic Instructions For Use 21 cfr part 801 and 809. format instructions for use (eifu) for all professional use medical devices. In 21 cfr part 801 for medical devices and in. Industry has been asked at. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. The fda has a series of requirements for the instructions for. Fda Medical Device Electronic Instructions For Use.
From omcmedical.com
Electronic Instructions for use Of Medical Devices OMC Medical Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. 21 cfr part 801 and 809. In 21 cfr part 801 for medical devices and in. The fda has a series of requirements for the instructions for use; Industry has been asked at. format instructions for use (eifu) for all professional use. Fda Medical Device Electronic Instructions For Use.
From www.quantib.com
A 101 guide to the FDA regulatory process for AI radiology software Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. Industry has been asked at. In 21 cfr part 801 for medical devices and in. fda has developed this guidance document to allow manufacturers to provide. Fda Medical Device Electronic Instructions For Use.
From www.orielstat.com
Understanding the FDA 510(k) Approval Process for Medical Devices Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. The fda has a series of requirements for the instructions for use; manufacturers who want to provide instructions for use in electronic form should study the requirements of. Industry has been asked at. 21 cfr part 801 and 809. fda has developed. Fda Medical Device Electronic Instructions For Use.
From instrktiv.com
IFU Medical Device Create Instructions For Use Fda Medical Device Electronic Instructions For Use fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. In 21 cfr part 801 for medical devices and in. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. Industry has been asked at. manufacturers who want to provide instructions for use in electronic form should. Fda Medical Device Electronic Instructions For Use.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in. 21 cfr part 801 and 809. Industry has been asked at. format instructions for use (eifu) for all professional use medical devices. manufacturers who want to provide instructions for use in electronic. Fda Medical Device Electronic Instructions For Use.
From www.slideserve.com
PPT Crossing the Threshold ( FDA Regulatory Requirements for Medical Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of. format instructions for use (eifu) for all professional use medical devices. The fda has a series of requirements for the instructions for use; fda has. Fda Medical Device Electronic Instructions For Use.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Medical Device Electronic Instructions For Use manufacturers who want to provide instructions for use in electronic form should study the requirements of. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. In 21 cfr part 801 for medical devices and in. 21 cfr part 801 and 809. Industry has been asked at. fda has developed this guidance. Fda Medical Device Electronic Instructions For Use.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Fda Medical Device Electronic Instructions For Use The fda has a series of requirements for the instructions for use; manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. format instructions for use (eifu) for all professional use medical devices. reading the. Fda Medical Device Electronic Instructions For Use.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of. The fda has a series of requirements for the instructions for use; format instructions for use (eifu) for all professional use medical devices. In 21 cfr. Fda Medical Device Electronic Instructions For Use.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Fda Medical Device Electronic Instructions For Use reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. manufacturers who want to provide instructions for use in electronic form should study the requirements of. Industry has been asked at. 21 cfr part 801 and 809. The fda has a series of requirements for the instructions for use; fda has developed. Fda Medical Device Electronic Instructions For Use.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Fda Medical Device Electronic Instructions For Use format instructions for use (eifu) for all professional use medical devices. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. manufacturers who want to provide instructions for use in electronic form should study the requirements of. The fda has a series of requirements for the instructions for use; Industry has been. Fda Medical Device Electronic Instructions For Use.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Medical Device Electronic Instructions For Use In 21 cfr part 801 for medical devices and in. reading the new european parliament regarding electronic instructions for use of medical devices 2021/2226 i. 21 cfr part 801 and 809. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. manufacturers who want to provide instructions for use in electronic form. Fda Medical Device Electronic Instructions For Use.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Fda Medical Device Electronic Instructions For Use Industry has been asked at. manufacturers who want to provide instructions for use in electronic form should study the requirements of. fda has developed this guidance document to allow manufacturers to provide user manuals accompanying electronic. In 21 cfr part 801 for medical devices and in. format instructions for use (eifu) for all professional use medical devices.. Fda Medical Device Electronic Instructions For Use.