Impact Assessment Tools In Pharmaceutical Industry . impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. A systematic process for the assessment, control, communication, and review of risks to the. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the importance of impact assessments in pharmaceutical product lifecycle management.
from acure.io
impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. discover the importance of impact assessments in pharmaceutical product lifecycle management. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. A systematic process for the assessment, control, communication, and review of risks to the.
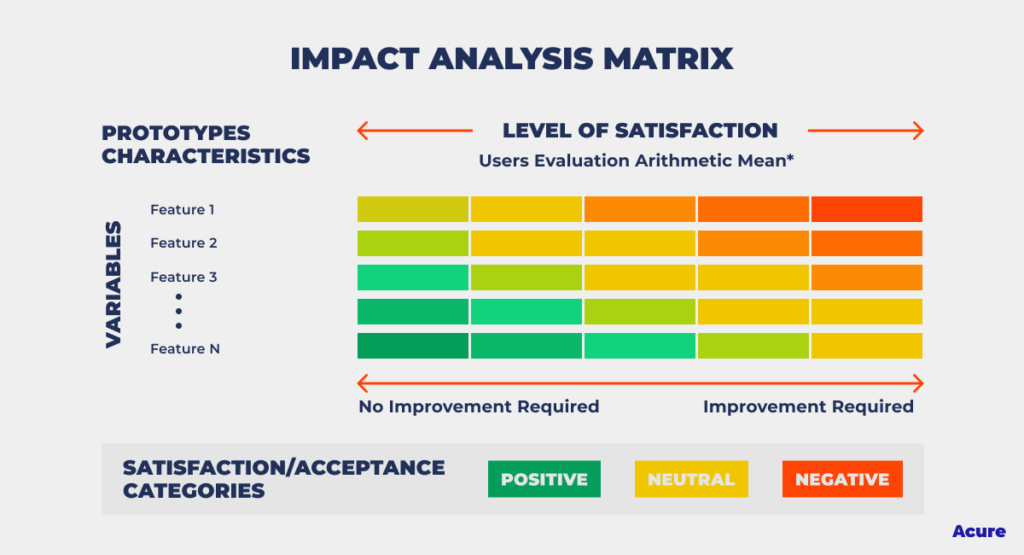
What is Impact Analysis? And Why Is It Important? Acure AIOps Platform
Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the importance of impact assessments in pharmaceutical product lifecycle management. A systematic process for the assessment, control, communication, and review of risks to the. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or.
From www.templatesdoc.com
44+ Free Impact Assessment Templates in Word Excel PDF Formats Impact Assessment Tools In Pharmaceutical Industry when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the importance of impact assessments in pharmaceutical product lifecycle management. A systematic process for the assessment, control, communication, and review of risks to the.. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. at step 2 of the ich process, a consensus draft text or guideline,. Impact Assessment Tools In Pharmaceutical Industry.
From polestarllp.com
8 Ways Pharmaceutical Companies Ensure Success With Analytics Impact Assessment Tools In Pharmaceutical Industry impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the importance of impact assessments in pharmaceutical product lifecycle management. This document provides practical guidance for gmp. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. A systematic process for the assessment, control, communication,. Impact Assessment Tools In Pharmaceutical Industry.
From www.researchgate.net
(PDF) Impact of Training in Pharmaceutical Industry An Assessment on Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control, communication, and review of risks to the. impact assessment is only. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. at step 2 of the ich process,. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. A systematic process for the assessment, control, communication, and review of risks to the. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the importance of impact assessments in pharmaceutical product lifecycle management.. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. when considering. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. impact assessment. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the importance of impact assessments in. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. A systematic process for the assessment, control, communication,. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. A systematic process for the assessment, control, communication, and review of risks to the. discover the importance of impact assessments. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the importance of impact assessments in pharmaceutical product lifecycle management. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control,. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. discover the. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the importance of impact assessments in pharmaceutical product lifecycle management. A systematic process for the assessment, control, communication, and review of risks to the. This document provides practical guidance for gmp inspectors when seeking to. Impact Assessment Tools In Pharmaceutical Industry.
From www.templatesdoc.com
44+ Free Impact Assessment Templates in Word Excel PDF Formats Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control, communication, and review of risks to the. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a.. Impact Assessment Tools In Pharmaceutical Industry.
From acure.io
What is Impact Analysis? And Why Is It Important? Acure AIOps Platform Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. A systematic process for the assessment, control, communication, and review of risks to the. impact assessment is only about, “whatever root cause is there. Impact Assessment Tools In Pharmaceutical Industry.
From www.ocmsolution.com
Best Change Control in Pharma Guide with Free Template & Tool OCM Impact Assessment Tools In Pharmaceutical Industry impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the importance of impact assessments in pharmaceutical product lifecycle management. when considering which quality systems could. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry A systematic process for the assessment, control, communication, and review of risks to the. discover the importance of impact assessments in pharmaceutical product lifecycle management. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that.. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry A systematic process for the assessment, control, communication, and review of risks to the. discover the importance of impact assessments in pharmaceutical product lifecycle management. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that.. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry A systematic process for the assessment, control, communication, and review of risks to the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Initial Risk Assessment Of Manufacturing Process Steps Pharmaceutical Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. A systematic process. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. A systematic process for the assessment, control, communication, and review of risks to the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance for gmp inspectors when seeking to. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. A systematic process for the assessment, control, communication, and review of risks to the. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. impact assessment is only about, “whatever. Impact Assessment Tools In Pharmaceutical Industry.
From www.ocmsolution.com
Best Process Impact Assessment Toolkit Template & Reporting Dashboard Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. at step 2 of the ich process, a consensus draft text. Impact Assessment Tools In Pharmaceutical Industry.
From www.templatesdoc.com
44+ Free Impact Assessment Templates in Word Excel PDF Formats Impact Assessment Tools In Pharmaceutical Industry This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry A systematic process for the assessment, control, communication, and review of risks to the. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance. Impact Assessment Tools In Pharmaceutical Industry.
From www.ocmsolution.com
Best Change Impact Assessment Tool, Templates & Reporting OCM Solution Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. A systematic process for the assessment, control, communication, and review of risks to the. impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. at step 2 of the ich process, a consensus draft text or guideline, agreed by. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control, communication, and review of risks to the. at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. This document provides practical guidance. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry impact assessment is only about, “whatever root cause is there is impacting our product quality directly or. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control, communication, and review of risks to the. This document provides practical guidance for gmp inspectors when seeking. Impact Assessment Tools In Pharmaceutical Industry.
From www.slideteam.net
Environmental Impact Assessment For A Pharmaceutical Company Case Impact Assessment Tools In Pharmaceutical Industry discover the importance of impact assessments in pharmaceutical product lifecycle management. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. This document provides practical guidance for gmp inspectors when seeking to evaluate the effectiveness of a. impact assessment is only about, “whatever root cause is there is impacting our. Impact Assessment Tools In Pharmaceutical Industry.
From www.smartsheet.com
Business Impact Analysis Toolkit Smartsheet Impact Assessment Tools In Pharmaceutical Industry at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is. discover the importance of impact assessments in pharmaceutical product lifecycle management. when considering which quality systems could benefit from a formalized quality risk management (qrm) component, systems that. A systematic process for the assessment, control,. Impact Assessment Tools In Pharmaceutical Industry.