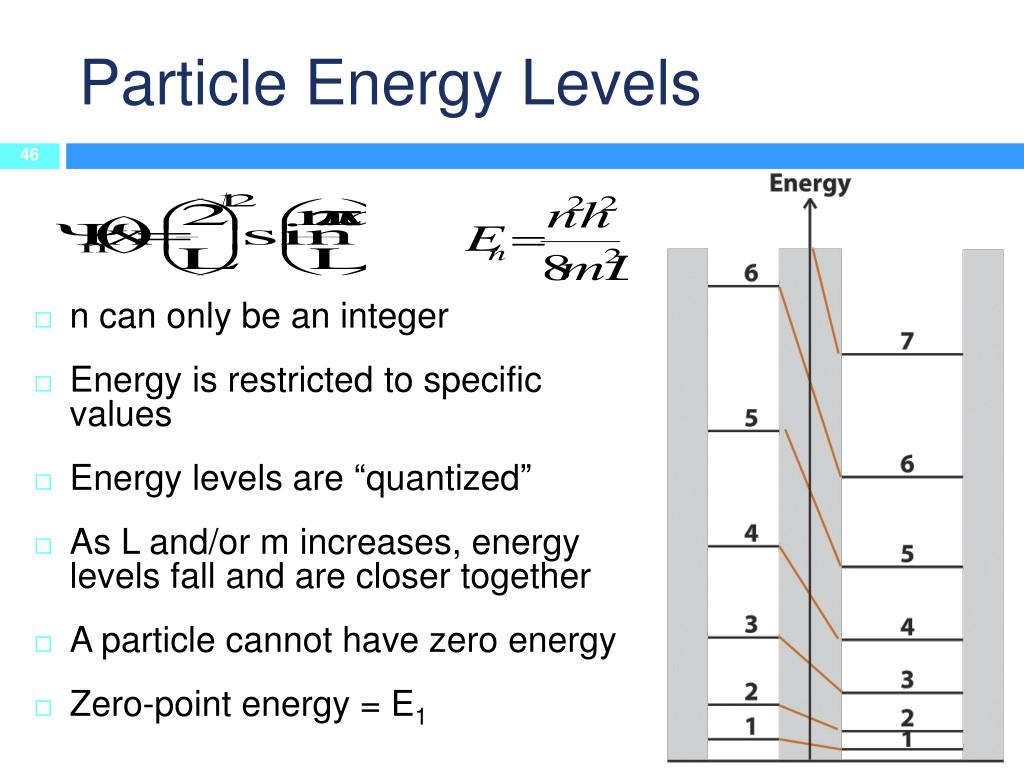
What Is The Particle Energy Level In A Liquid . molecules in a liquid have more energy than molecules in a solid. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. As a result, the particles in a liquid move faster in. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. And if you heat it up even more, the molecules will speed up so. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom.
from www.slideserve.com
particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. And if you heat it up even more, the molecules will speed up so. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. molecules in a liquid have more energy than molecules in a solid. As a result, the particles in a liquid move faster in. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from.
PPT CHEMISTRY XL14A NATURE OF LIGHT AND THE ATOM PowerPoint
What Is The Particle Energy Level In A Liquid And if you heat it up even more, the molecules will speed up so. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. molecules in a liquid have more energy than molecules in a solid. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up even more, the molecules will speed up so. As a result, the particles in a liquid move faster in. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. molecules in a liquid have more energy than molecules in a solid. the particles of a liquid have enough energy to break free of some. What Is The Particle Energy Level In A Liquid.
From diagramlistreporting.z14.web.core.windows.net
Diagram Of Particles In Solid Liquid And Gas What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up even more, the molecules will speed up so. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. the particles in. What Is The Particle Energy Level In A Liquid.
From www.snexplores.org
Explainer What are the different states of matter? What Is The Particle Energy Level In A Liquid molecules in a liquid have more energy than molecules in a solid. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. And if you heat it. What Is The Particle Energy Level In A Liquid.
From www.britannica.com
phase Definition & Facts Britannica What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. And if you heat it up even more, the molecules will speed up so. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. the particles in a liquid have more kinetic energy than the particles in the. What Is The Particle Energy Level In A Liquid.
From www.shutterstock.com
Theory Matter Infographic Diagram Showing Stock Illustration What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases.. What Is The Particle Energy Level In A Liquid.
From courses.lumenlearning.com
The Dissolution Process Chemistry What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. And if you heat it up even more, the molecules will speed up so. the particles in a liquid have more kinetic energy than the particles in the. What Is The Particle Energy Level In A Liquid.
From giouyvaim.blob.core.windows.net
Do Solids And Liquids Expand When Heated at Frank Gibbs blog What Is The Particle Energy Level In A Liquid if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. And if you heat it up even more, the molecules will speed up so. As a result, the particles in a liquid move faster in. the energy level increases as a substance moves from solid to liquid,. What Is The Particle Energy Level In A Liquid.
From wiredatasleflyneomoi6.z22.web.core.windows.net
Solid Liquid And Gas Particle Diagram What Is The Particle Energy Level In A Liquid molecules in a liquid have more energy than molecules in a solid. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. the energy level. What Is The Particle Energy Level In A Liquid.
From www.docbrown.info
GASES LIQUIDS SOLIDS States of Matter, particle theory models What Is The Particle Energy Level In A Liquid particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. As a result, the particles in a liquid move faster in. molecules in a liquid have more energy than molecules in a solid. the particles in a liquid have more kinetic energy than the particles in the. What Is The Particle Energy Level In A Liquid.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Is The Particle Energy Level In A Liquid if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. particles have no kinetic energy at all so no energy can be removed and the temperature. What Is The Particle Energy Level In A Liquid.
From socratic.org
How does the arrangement of the particles in a liquid compare to that What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. And if you heat it up even more, the molecules will speed up so. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. molecules. What Is The Particle Energy Level In A Liquid.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions What Is The Particle Energy Level In A Liquid molecules in a liquid have more energy than molecules in a solid. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. As a result, the particles in a liquid move faster in. the energy level of liquid is typically higher than that of solids due to. What Is The Particle Energy Level In A Liquid.
From www.slideserve.com
PPT Chapter 2 A Particle View of Matter PowerPoint Presentation What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. As. What Is The Particle Energy Level In A Liquid.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. particles have no kinetic energy at all so no energy can be removed and the temperature. What Is The Particle Energy Level In A Liquid.
From exyedgutk.blob.core.windows.net
Zn Element Energy Levels at Wendy Arwood blog What Is The Particle Energy Level In A Liquid molecules in a liquid have more energy than molecules in a solid. As a result, the particles in a liquid move faster in. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. the energy level increases as a substance moves from solid to liquid, and further. What Is The Particle Energy Level In A Liquid.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. As a result, the particles in a liquid move faster in. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up. What Is The Particle Energy Level In A Liquid.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement What Is The Particle Energy Level In A Liquid And if you heat it up even more, the molecules will speed up so. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. As a result, the particles in a liquid move faster in. molecules in a liquid have more energy than molecules in a solid. . What Is The Particle Energy Level In A Liquid.
From www.researchgate.net
Calculated single particle energy levels in the 1ℎ 11/2 subshell What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. particles have no kinetic. What Is The Particle Energy Level In A Liquid.
From www.slideserve.com
PPT Energy and States of Matter PowerPoint Presentation, free What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up even more, the molecules will speed up so. As a result, the particles in a liquid move faster in. the energy level increases as a substance moves from solid to liquid, and. What Is The Particle Energy Level In A Liquid.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the particles in a liquid have more kinetic energy than the particles in the corresponding. What Is The Particle Energy Level In A Liquid.
From www.freeexamacademy.com
The Particulate Nature Of Matter IGCSE Chemistry Free Exam Academy What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy. What Is The Particle Energy Level In A Liquid.
From www.slideshare.net
Particle Theory (Slg Introduction) What Is The Particle Energy Level In A Liquid if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. And if you heat it up even more, the molecules will speed up so. the particles in. What Is The Particle Energy Level In A Liquid.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock What Is The Particle Energy Level In A Liquid particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. molecules in a liquid have more energy than molecules in a solid. And if you heat it up even more, the molecules will speed up so. the particles in a liquid have more kinetic energy than the. What Is The Particle Energy Level In A Liquid.
From sciencenotes.org
States of Matter What Is The Particle Energy Level In A Liquid the particles in a liquid have more kinetic energy than the particles in the corresponding solid. And if you heat it up even more, the molecules will speed up so. molecules in a liquid have more energy than molecules in a solid. the energy level of liquid is typically higher than that of solids due to the. What Is The Particle Energy Level In A Liquid.
From studyrocket.co.uk
The Particle Model GCSE Chemistry Science) OCR Revision What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. molecules in a liquid have more energy than molecules in a solid. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. particles have no kinetic energy at all so no energy can be removed. What Is The Particle Energy Level In A Liquid.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. molecules in a liquid have more energy than molecules in a solid. particles have no kinetic energy at all so no energy can be removed and. What Is The Particle Energy Level In A Liquid.
From www.slideserve.com
PPT Material since exam 3 PowerPoint Presentation, free download ID What Is The Particle Energy Level In A Liquid As a result, the particles in a liquid move faster in. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. if you add heat energy. What Is The Particle Energy Level In A Liquid.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up even more, the molecules will speed up so. molecules in a liquid have more energy than molecules in a solid. As a result, the particles in a liquid move faster in. . What Is The Particle Energy Level In A Liquid.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. As a result, the particles in a liquid move faster in. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. molecules in a liquid have more energy than molecules in. What Is The Particle Energy Level In A Liquid.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation What Is The Particle Energy Level In A Liquid if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. molecules in a liquid have more energy than molecules in a solid. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. the energy level of. What Is The Particle Energy Level In A Liquid.
From www.coursehero.com
[Solved] (a) Diagram 2 shown above represents a particlelevel view of What Is The Particle Energy Level In A Liquid the particles in a liquid have more kinetic energy than the particles in the corresponding solid. And if you heat it up even more, the molecules will speed up so. molecules in a liquid have more energy than molecules in a solid. the energy level of liquid is typically higher than that of solids due to the. What Is The Particle Energy Level In A Liquid.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind What Is The Particle Energy Level In A Liquid particles have no kinetic energy at all so no energy can be removed and the temperature cannot get any lower. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. As a result, the particles in a liquid move faster in. the energy level of liquid. What Is The Particle Energy Level In A Liquid.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Is The Particle Energy Level In A Liquid molecules in a liquid have more energy than molecules in a solid. the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. if you add. What Is The Particle Energy Level In A Liquid.
From www.slideserve.com
PPT CHEMISTRY XL14A NATURE OF LIGHT AND THE ATOM PowerPoint What Is The Particle Energy Level In A Liquid the particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. the particles in a liquid have more kinetic energy than the particles in the corresponding solid. if you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy. What Is The Particle Energy Level In A Liquid.
From www.teachoo.com
Characterstics of Particles of Matter Class 9 Science Notes Teacho What Is The Particle Energy Level In A Liquid the energy level of liquid is typically higher than that of solids due to the increased molecular motion and freedom. And if you heat it up even more, the molecules will speed up so. the energy level increases as a substance moves from solid to liquid, and further increases as it transitions from. As a result, the particles. What Is The Particle Energy Level In A Liquid.